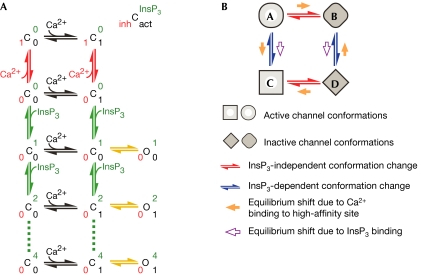
Figure 3.
Kinetic schemes accounting for the observed InsP3R channel response latencies. (A) A qualitative model stipulating distinct high-affinity inhibitory and activating Ca2+ sites in each InsP3R channel. C and O represent closed inactive and active channel conformations. Channel conformations with different ligand binding states are explicitly shown in the scheme by superscript and subscript numbers in different colours: number of occupied high-affinity Ca2+ inhibitory sites in red, occupied high-affinity activating Ca2+ sites in black and occupied InsP3 sites in green (as indicated by the key at the top right). Ligand binding and dissociation are indicated by arrows: InsP3 binding in green, Ca2+ binding to inhibitory sites in red and Ca2+ binding to activating sites in black. Yellow arrows represent conformation changes. Because the channel is a tetrameric unit, more than one Ca2+ can bind to either the activating or inhibitory sites. Those ligand-binding states are not shown in the scheme for clarity. Multiple InsP3 binding to the channel (dashed lines) is shown to demonstrate that the scheme can accommodate branched Markov chains. (B) A qualitative allosteric model stipulating only one kind of high-affinity Ca2+ site in an InsP3R channel. Occupation of the InsP3 binding sites causes the channel to adopt the C and D conformations instead of the A and B conformations, so that Ca2+ binding to the high-affinity Ca2+ site becomes activating instead of inhibitory. Unlike (A), only conformation changes are shown in this kinetic scheme. InsP3 and Ca2+ binding to the channel, and the ligand-binding state of the channel conformations are omitted for clarity.