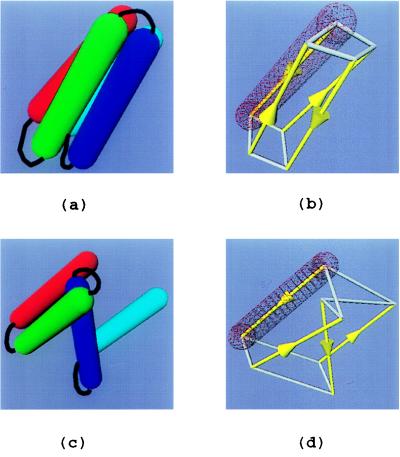
Figure 1.
Four-helix bundle protein model. (a) Folded protein. (b) Schematic drawing of a. Helix major axes, connecting the center of hemispherical caps, are in yellow (arrows point to N terminus). The two quadrilaterals that are formed by the helix ends are outlined in white. The length of the longest edge of the two quadrilaterals, d, is used as the folding criterion, as well as the guideline for splitting and combining WEB particles. The wire-framed rendition of the exclusion volume of the first helix (red) is shown for comparison. In this case, d = 10Å. (c) Unfolded protein. (d) A schematic drawing of c, showing major axes and distances between helix ends (d = 27.2 Å).