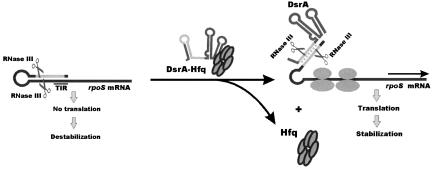
FIGURE 4.
Model for post-transcriptional regulation of rpoS expression by RNase III. The translation initiation region (TIR) of the E. coli rpoS mRNA is embedded into a complex secondary structure, thereby preventing efficient ribosome binding. RNase III can cleave within the double-stranded segment of this structure, which results in destabilization of the transcript in the wild-type E. coli strain when compared to its isogenic counterpart lacking functional RNase III. In contrast to limited synthesis of RpoS under normal growth conditions, the level of RpoS is up-regulated in the presence of increasing amounts of DsrA, which accumulate at low temperature. By base-pairing with the complementary region of the rpoS leader, DsrA disrupts the inhibitory secondary structure, thereby facilitating ribosome loading and subsequent translation of RpoS (Repoila et al. 2003). DsrA/rpoS duplex formation, which is facilitated by the RNA chaperone Hfq, not only abrogates RNase III cleavage within the rpoS leader at positions (−94/−15), but also creates a new RNase III cleavage site within the DsrA/rpoS duplex. RNase III cleavage at this site prevents reuse of DsrA for multiple cycles of rpoS activation. Moreover, the main body of the DsrA-activated rpoS mRNA is covered by translating ribosome and therefore protected from degradation by E. coli ribonucleases.