Abstract
The surrogate light chain (SLC) is a key regulator of B cell development in the bone marrow, resulting in mature B cells that produce antibodies that are capable of interacting with antigens. The SLC comprises two noncovalently interacting proteins: VpreB and 14.1. We engineered a construct to represent the complete immunoglobulin-like domain of the SLC variable domain in a single protein chain that could be bacterially expressed. In this construct, the incomplete immunoglobulin domain of VpreB (residues 1–102) was linked to the J-segment of 14.1 (residues 40–53), which provided one β-strand to complete the V-like domain (VpreBJ). Because VpreBJ has the interface to VH chains, but lacks the unique region of 14.1, which is important for SLC signaling, we predict that a properly folded VpreBJ would have the potential to act as a dominant negative mutant of the surrogate light chain. X-ray crystallography of VpreBJ at 2.0 Å resolution showed that the engineering was successful. With its two β-pleated sheets, packed face-to-face, the single chain VpreBJ resembles a mature light chain immunoglobulin V-domain (VL). The surface that would normally interact with the VH chain interacts with a crystallographically related VpreBJ molecule. The presence of dimeric species in solution was verified by analytical ultracentrifugation. VpreBJ is easily overexpressed in bacteria, while retaining the native conformation of an immunoglobulin domain, and thus may serve as an important reagent for future studies in B-cell development.
Keywords: protein structures—new; protein engineering, proteins of the immune system; crystallography; heteronuclear NMR; B-cell development; protein–protein interactions; immunoglobulin
The ability of the human immune system to fight infectious diseases is the result of the successful interaction of cells and proteins of the immune system. Mature B cells are capable of secreting antibodies and expressing B cell receptors on their cell surfaces. The great diversity of heavy (H) and light (L) chain variable domains (VH and VL) enables the recognition of many pathogens.
The development of B lymphocytes from hematopoietic stem cells can be divided into distinct stages, based on the sequential expression or loss of cell surface or intercellular proteins (Rolink et al. 1999). The differentiation of precursor cells into pro-B, pre-B, and immature B cells is linked to the successive rearrangement of H and L chains and results in the development of the immunoglobulin repertoire (Tonegawa 1983). In the pro-B cell to pre-B cell transition, successfully rearranged H chains are coexpressed with a L chain-like molecule called the surrogate light chain (SLC). There is only one human SLC to pair with the entire pool of H chains generated by gene rearrangement. The proposed function of the SLC is to test a μH chain for its ability to bind a conventional L chain that will be expressed later in the development of the B cell (Kline et al. 1998).
The SLC consists of two proteins, VpreB and 14.1, which are noncovalently linked and form a λ light chain-like structure. In contrast to mature VLs, VpreB features an incomplete Ig v-domain that requires one β-strand from 14.1 for completion of one of its β-sheets (Minegishi et al. 1999). Both VpreB and 14.1 contain sequences that have no homology with Igs, called unique regions, which are C-terminal in VpreB and N-terminal in 14.1 (U-VpreB and U-14.1). The unique region of 14.1 is important for phosphorylation and signaling of the pre-BCR (Ohnishi and Melchers 2003), which was proposed to occur via pre-BCR aggregation on the cell surface and interaction with extracellular matrix components like galectin-1 (Rossi et al. 2006) and stroma cell-associated heparan sulfate (Bradl et al. 2003).
In order to engineer a tool to test pre-BCR signaling, we generated a construct that lacks the unique regions of both VpreB and 14.1. In our single chain representation of the Ig domain of the SLC variable region (VpreBJ), the incomplete Ig domain of VpreB (residues 1–102) was completed by covalent linkage of one β-strand from the J-segment of 14.1 (residues 40–53). Because VpreBJ comprises the complete β-sheet interface to VH heavy chains, but lacks the unique region of 14.1, which has been shown to mediate signaling, we predict that VpreBJ will bind μH chains, but not signal, and therefore act as a dominant negative mutant of SLC.
The structure of noncovalently linked VpreB and 14.1 in complex with a μH chain was recently solved at 2.7 Å resolution using proteins expressed in insect cells (Bankovich et al. 2007). The structure of the bacterially expressed engineered single chain fusion of VpreB and 14.1 (VpreBJ) presented here is of higher resolution but closely resembles that of the pre-BCR, which demonstrates that VpreBJ is properly folded, and therefore may serve as an important tool for further immunological investigation.
Results and Discussion
Domain engineering and bacterial expression
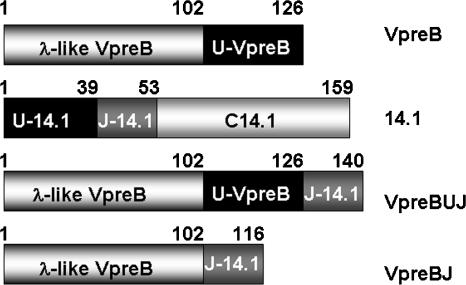
In vivo folding studies of transiently expressed proteins in COS7 cells had indicated that complementation of the incomplete Ig domain of VpreB by the extra β-strand of the J-segment of 14.1 is necessary and sufficient for folding and assembly of the surrogate light chain V-domain (Minegishi et al. 1999). Based on these findings, we engineered the human SLC variable domain by fusing the Ig domain of VpreB (residues 1–102) to the J-segment of 14.1 (residues 40–53). The length of the loop was designed to match the minimal requirement for CDR3 loops observed in light chains. This resulted in the complete Ig domain of the SLC variable domain being encoded in a single protein that lacks the unique regions of both VpreB and 14.1. We also designed a second construct, VpreBUJ, containing the unique region of VpreB, located between the Ig domain of VpreB and the J-domain of 14.1, but not C-terminal as in the construct expressed in fibroblasts (Minegishi et al. 1999). The domain structures of VpreB, 14.1, VpreBUJ, and VpreBJ are shown in Figure 1.
Figure 1.
Domain structures of the proteins comprising the human SLC, VpreB, and 14.1, and of our constructs VpreBUJ and VpreBJ. In VpreB, an N-terminal λ Ig-like variable domain (residues 1–102), which lacks one β-strand, is followed by a region without homology with other proteins, U-VpreB (residues 103–126). 14.1 contains an N-terminal unique region, U-14.1 (residues 1–39), followed by a J-segment (residues 40–53), which provides a β-strand that completes the Ig-like domain of VpreB, and a C-terminal constant domain of high homology with mature light-chain constant domains (residues 54–159). In the construct VpreBUJ, the sequence of VpreB (residues 1–126) is linked to the J-segment (127–140), while in VpreBJ, the Ig-like domain of VpreB alone (residues 1–102) is linked to the J-segment, comprising a complete Ig domain representing the V domain of the SLC.
Sequences were linked to a Staphylococcus aureus Protein A (SPA) tag for purification, and expressed in Escherichia coli BL21(DE3)pLysE cells. Because of the secretion signal of the vector, VpreBJ and VpreBUJ were secreted into the medium, with a typical yield of 5 mg and 7 mg of purified protein from one liter of culture. A thrombin cleavage site between VpreBJ or VpreBUJ and the SPA tag enabled selective thrombin digestion, which was followed by gel filtration and yielded VpreBJ plus the sequence AAAHGLVPR from the cloning vector. The identity and purity of the proteins were analyzed by denaturating polyacrylamide gel electrophoresis (SDS-PAGE) and mass spectroscopy and had the expected molecular masses of 13.87 kDa for VpreBJ and 16.81 kDa for VpreBUJ.
Crystallization and structure determination of VpreBJ
Large, hexagonal prism-shaped crystals of VpreBJ were obtained by vapor diffusion (see Materials and Methods). The crystals diffracted to 2.0 Å resolution and the structure of VpreBJ was solved by molecular replacement using the human λ light chain from the patient mcg (PDB file 2MCG) as a model. The refined structure VpreBJ consists of 116 amino acids: the Ig domain of VpreB (residues 3–102), residues 103–116 of 14.1, and two C-terminal alanines from the vector sequence. The structure was refined to 2.0 Å resolution to a crystallographic R factor of 0.18 and an R free value of 0.23. The model has good geometry (Table 1), and all residues except one are in the allowed region of the Ramachandran plot.
Table 1.
Data collection and refinement statistics
The structure of the VpreBJ monomer shows an Ig-like fold (Fig. 2A) with two β-sheets, packed face-to-face and covalently connected by a disulfide bond. As in VL domains, the four-strand and five-strand β-sheets of VpreBJ are formed by the ABDE and GFCC′C″ strands. A β-bulge produces a rotation in strand G (Fig. 2A), which is conserved with that described in some VL domains (Chothia et al. 1998). Similar to VL domains, the five-stranded β-sheet of VpreBJ consists of sequence elements to which CDR regions contribute significantly (Fig. 3). While the strand interface of VpreBJ closely resembles that of VL domains, the CDR2 region, which contains the strands C′ and C″, is significantly longer, having 11 amino acids instead of 7. The elongated CDR2 region of VpreBJ (Fig. 2B), which has also been observed in human germ-line V λ chain subgroup 5 and in nonmammalian species such as horned shark and frog, has been proposed to extend the SLC interface with VH chain (Solomon et al. 1997). In support of this model, the CDR2 loop of VpreB was found to interact with the CDR3 loop of VH (Bankovich et al. 2007).
Figure 2.
The structure of VpreBJ has an immunoglobulin-like fold. (A) Ribbon representation. Strand G, in red, is provided by the J-segment of 14.1 that completes the immunoglobulin-like domain of VpreB. The CDR1 loop links strand B (blue), from the outer-face β-sheet to strand C (cyan) of the inner-face β-sheet; the CDR2 loop links the strands C′ and C″ (green) of the extended CDR2 region, which is composed of 11 amino acids rather than the usual 7; and the engineered “CDR3” loop links strand F (orange) to strand G (in red). The β-sheet facing the viewer, comprising the five β-strands, is predicted to make up the interface to VH. (B) α-Carbon representation of VpreBJ (in green), superimposed on three representative human VL domains (PDB accession nos.: 1ADQ, 2MCG, and 1W72, in blue, magenta, and yellow, respectively). Of significance is the CDR2 loop of VpreBJ, which is more extended than that of the VL and which overlap closely in both the CDR2 region and sheet interface. (C) Interface residues of the VpreBJ dimer. The interface to the VH domain, where bulky hydrophobic residues protrude from β-strands. Nine of the VpreBJ interface residues are shown (different shades of magenta), which are compared with VL residues in Table 2. Y36, Y38, and Q40 (green), the latter of which forms hydrogen bonds across the dimer interface, originate from strand C. P46 and F48 originate from strand C′. Y95 and A97 originate from strand F, F106 originates from strand G, and G99 originates from the CDR3 loop, which we engineered by linking VpreB with the J-domain of 14.1. (D) View of the hydrophobic cavity at the interface of the VpreBJ dimer (cyan and blue), where aromatic residues pack in a characteristic “herringbone” motif. The residues Y36, Y38, Q40, P46, F48, A97, G99, Y95, and F106 are shown in relationship to the symmetry-related molecule.
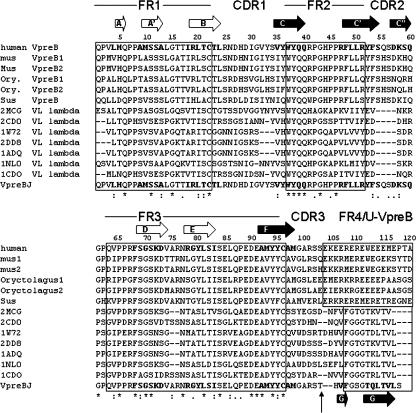
Figure 3.
Alignment of human VpreB and VpreB sequences from mammalian species, human VL sequences, and the VpreBJ sequence. The location of secondary structure elements are indicated with respect to the amino acid sequence of VpreBJ. Framework regions (FR1–4), and the unique region of VpreB are shown in boxes that are connected by complementary determining regions (CDR1–CDR3). A vertical arrow indicates the beginning of the J-segment in VpreBJ. The strands are lettered according to VL domains (Chothia et al. 1985). Black arrows indicate the β-strands that comprise an interface to VH and make up the inner-face sheet, whereas white arrows show β-strands that do not (outer-face sheet). The first six lines of the alignment show human VpreB aligned to the VpreB from other mammalian species (mouse, rabbit, and pig). The following lines show the alignment of our construct VpreBJ, bottom, to the seven human VL chains of known structure that have the highest homology. The sequences were aligned using ClustalW (Thompson et al. 1994) and manually adjusted for structural homology. (*) 100% conservation; (:) conservation or conservative substitutions; (.) both to a lesser degree.
The VpreBJ crystallographic dimer mimics VL–VH interactions
In the VpreBJ crystal, a twofold axis positions two VpreBJ monomers in close association, allowing the investigation of their interface. Because Bence-Jones proteins, which are light chain dimers, associate in the same manner as the VL–VH module (Schiffer et al. 1973), and side chain arrangements at the VL dimer interface mimic side chain arrangements at the VL–VH interface (Novotny and Haber 1985), the interface of the observed VpreBJ dimer is a good model for the interaction of VpreBJ with VH, as it occurs in the pre-BCR.
As in VL–VH, the relative orientation of the domains in the VpreBJ dimer is tilted, so that at the very bottom of the dimer, the slight separation of the two molecules results in a small cavity filled with ordered water molecules. The cavity ends when two hydrogen bonds formed between residues Q40 in each monomer anchor the interface β-sheets in their relative orientation across the domain–domain interface. This glutamine hydrogen bond is conserved in some VL–VH interactions. The center of the interface consists of residues protruding from the five-stranded β-sheet and is highly hydrophobic (Fig. 2C,D). Bulky residues, Y36, Y38, P46, F48, Y95, and F106 contribute to the large nonpolar interface of 809 Å2 per monomer, which is comparable to the average interface area of a VL–VH interaction of 825 Å2 (Bankovich et al. 2007).
Ten residues located on β-strands of VL are important for the interaction with VH (Table 2; Chothia et al. 1985). Nine corresponding residues of VpreBJ are shown (Fig. 2C,D), while the tenth residue, H104, is not, because its proximity to the engineered covalent junction between VpreB and 14.1 is likely to be the cause for its altered side chain conformation. Of the 10 residues, VpreB contributes eight, 14.1 two, and half are different in VpreBJ compared with VL. Residues Y38, Q40, and P46 are conserved both in mammalian VpreB and in VL, while residue Y95 is either conserved in mammalian VpreB, or a conservative replacement, as phenylalanine in pig VpreB (Fig. 3). Interestingly, F48 is conserved in VpreB, but different in VL, where a smaller hydrophobic residue occupies this position, either leucine (in 12 of 17 VL), threonine, or valine. Unlike the smaller residue of the VL, F48 of VpreB contributes to the herringbone motif of bulky hydrophobic residues and reaches far into the interface with VH, resulting in a different VH interaction of VpreB compared with VL. A second striking difference is seen at position 99, where the G conserved in VpreB is replaced in VL either by Y or W. The switch to a bulky hydrophobic residue suggests different interactions of VpreBJ and VL in VH binding. An understanding of the consequences of these replacements would require structure determination of the same VH domain with VL and with the SLC variable domain.
Table 2.
Comparison of VpreBJ interface residues with the corresponding VL residues identified as important in VH interaction (Chothia et al. 1985)
A monomer–dimer equilibrium in solution
To determine the extent to which the dimer seen in the crystal structure of VpreBJ exists in solution, we performed sedimentation equilibrium analytical ultracentrifugation (AUC) experiments (Fig. 4). The data obtained under all conditions could be fitted to both an ideal single species model and a monomer–dimer equilibrium. When fitted to an ideal single species model, an average apparent molecular mass of 20,417 ± 97 Da was obtained, which is in-between the mass of the monomer (13,878 Da) and the mass of the dimer (27,756 Da). The dissociation constant, Kd, extracted from fitting to a monomer–dimer model, was determined to be 34.3 ± 2.2 μM using data obtained at two different rotor speeds (18,000 and 25,000 rpm) and two different concentrations (20 and 30 μM). Figure 4 shows a representative trace, where the results from identical experiments collected in triplicate gave an average Kd of 38.4 μM. Overall, the Kd is weaker than known VH–VL associations (Klein et al. 1979). The existence of a dimer is consistent with the hydrophobic interface observed between subunits, enabling dimerization in an aqueous solution. Analysis of 2D 15N, 1H HSQC NMR spectra showed that the number of N-H cross-peaks in the well-dispersed regions exceeded the number of residues, and analysis of HNCOCACB and HNCACB 3D NMR experiments identified twice as many glycine–serine pairs in the spectra as in the sequence, further supporting the existence of a monomer–dimer equilibrium for VpreBJ.
Figure 4.
Analytical ultracentrifugation using sedimentation equilibrium shows the presence of a dimer in solution. A representative trace for VpreBJ at the concentration of 30 μM and rotor speed of 18,000 rpm is shown. The solid line is fit to the data and residuals are shown above. Under these conditions, an average Kd of 38.4 μM was obtained using three data sets. Over all conditions, the Kd was 34.3 ± 2.2 μM.
The structure of the unique region of VpreB
The X-ray structure of the pre-BCR, 2H32, showed significant disorder in both unique regions of 14.1 and VpreB, where only 12 of the 24 amino acids of the unique region of VpreB and only three of 39 amino acids of the unique region of 14.1 could be traced (Bankovich et al. 2007). To investigate the unique region of VpreB (residues 103–126), we compared 15N, 1H HSQC NMR spectra obtained from VpreBUJ, which contains the unique domain of VpreB, and VpreBJ, which does not (Supplemental Fig. S1). The flexibility of the unique region of VpreB is indicated by peaks in the center of the spectrum of VpreBUJ, which is characteristic for peptides in random conformation. These peaks were mostly absent in the spectrum obtained from VpreBJ, thus attributing these peaks to the unique region of VpreB. Most of the well-dispersed cross-peaks did not change position, suggesting that the integrity of the Ig domain was retained in the absence of the unique region, a fact that was confirmed by the high degree of superimposition of the structure of VpreBJ with the structure of the pre-BCR (Fig. 5).
Figure 5.
(A) Superimposition of VpreBJ to parts of the structure of the pre-B cell receptor (2H32). VpreBJ is shown in magenta, with the CDR regions distinguished in different colors: CDR1 in blue, CDR2 in yellow, and the CDR3 loop introduced by linking VpreB to the J-domain of 14.1 in light green. For 2H32, VpreB and the J-domain of 14.1 are shown in cyan, while parts of the unique regions of VpreB and 14.1 are shown in dark green. (B) Close-up of the J-segment of 14.1 from 2H32, magenta, superimposed on the J-segment of 14.1 from VpreBJ, green. A portion of the Ig domain of VpreB, from VpreBJ, linked to the J-segment, is in blue. Two residues of the unique region of 14.1 are shown in light green. F106 of VpreBJ overlaps closely with F61 from 2H32, while H104 of VpreBJ is different from H59 of 2H32.
The structure of the CDR3 region of VpreBJ
VpreBJ superimposes well with 2H32 except for five amino acids of VpreBJ adjacent to the covalent linkage between VpreB and 14.1. In 2H32, H104 faces toward the antigen-binding site, while in VpreBJ, it is oriented toward the VH-interface. For the remaining common sequence, the close overlap with 2H32 shows that for VpreBJ, the loop corresponding to CDR3 of VL chains correctly positions strand G, provided by the J-segment of 14.1, within the incomplete β-sheet of the Ig domain of VpreB. Clearly, F106, which originates from strand G of VpreBJ, superimposes with F61 from 14.1 of 2H32 (Fig. 5B), confirming that in the engineered protein VpreBJ, the J-domain of 14.1 adopts its native conformation.
In this work we show that covalent linking of the incomplete Ig domain of VpreB with one β-strand of the J-segment from 14.1 results in a complete Ig domain. In VpreBJ, the loop that links the two protein chains and corresponds to CDR3 of VL is directed toward the equivalent of an antigen-binding site, where the addition of the unique regions of VpreB and 14.1 would clearly leave little space for antigen binding in a pre-BCR complex. This conclusion is consistent with the crystal structure of pre-BCR, where 12 and 3 amino acids of the unique regions of VpreB and 14.1, respectively, filled the equivalent of an antigen-binding site (Bankovich et al. 2007).
Stable association of the SLC with the μVH domain requires a complete Ig domain of VpreB, but neither the constant domain of 14.1 nor the unique regions of VpreB and 14.1 (Minegishi et al. 1999). In contrast, the unique region of 14.1 is indispensable for signaling through the pre-B cell receptor (Ohnishi and Melchers 2003). In mice, deletion of the unique region of λ5, which corresponds to 14.1 of the human pre-BCR, resulted in increased cell-surface representation, a diminished rate of aggregation and internalization, and abolished tyrosine phosphorylation, indicating a lack of signal transduction (Ohnishi and Melchers 2003). The single chain VpreBJ created here, which does not include the unique region of 14.1, is likely to be able to associate with a heavy chain in a pre-B cell receptor, but it is predicted not to signal and, therefore, to act as a dominant negative mutant that could be tested in a similar mouse model. We predict that expression of the single chain VpreBJ, instead of VpreB and λ5 as part of a reconstituted pre-BCR, would show differences in surface expression and tyrosine phosphorylation status and therefore abolished signaling. In the absence of the unique region of 14.1, an increased surface expression of the pre-BCR is expected because a slower rate of constitutive pre-BCR internalization was observed without a change in the level of protein expression in mutant pre-BCR (Ohnishi and Melchers 2003). At the same time, phosphorylation is likely to decrease, because whereas the pre-BCR seems to be constitutively phosphorylated, a mutant pre-BCR lacking the unique region of 14.1 has diminished phosphorylation (Ohnishi and Melchers 2003). Signaling through the pre-BCR is mediated by tyrosine phosphorylation. The fact that the structure of the single chain VpreBJ is the same as in the corresponding part of the native pre-BCR supports the likelihood that it will be a useful reagent for testing the role of signaling through the pre-BCR in certain leukemias and autoimmune diseases that are associated with aberrant pre-BCR function (Jumaa et al. 2005).
Materials and Methods
Expression and purification of human VpreBJ
The coding regions of human VpreB (residues 1–126), and the J-segment of 14.1 (residues 40–53) were inserted into the multicloning site of the bacterial plasmid vector pIg203 (Hirabayashi et al. 1995), N-terminal to a thrombin cleavage site and SPA tag for purification. The vector contains a T7 promoter and the secretion signal peptide of bacterial alkaline phosphatase (PhoA). The human VpreB exon, which was cloned from genomic DNA by PCR, included its unique region (U-VpreB) and was inserted after the PhoA sequence, followed by the J-segment of 14.1, resulting in the construct VpreBUJ. A second construct VpreBJ was designed using deletion primers to remove 24 residues of the unique region of VpreB from the VpreBUJ construct. The primer sequences used were 5′-ATGGGGGCCCGCAGCACGCATGTGTTTGGC-3′ and its complement.
E. coli BL21(DE3)pLysE cells were transformed with the constructs. A total of 0.75 L of LB medium was inoculated with 15 mL of overnight culture containing 50 μg/mL ampicillin and 34 μg/mL chloramphenicol and grown at 37°C with shaking at 200 rpm until the OD600nm reached 0.6–0.7. Protein expression was induced overnight at 25°C by addition of IPTG (isopropylthiogalactoside) to 0.5 mM. The medium, containing protein, was centrifuged and filtered though 0.45 μm cellulose acetate filters (Corning, Inc.). IgG beads (IgG Sepharose, 6 fast flow resin, GE-Healthcare BioSciences) equilibrated with buffer A (50 mM Tris, pH 7.5, 250 mM NaCl, 10% Glycerol, 0.2% NP40) were incubated with the supernatant, and successively washed five times with buffer A and then buffer B (50 mM Tris, pH 7.5, 250 mM NaCl). The protein was acid-eluted with 20 mM glycine (pH 2.5), and neutralized with 1 M Tris (pH 9). Fractions containing protein, which were detected photometrically at 280 nm, were pooled and adjusted to pH 7.5. The fusion protein was digested with 8 μg of thrombin (bovine α-thrombin, Hematologic Technologies, Inc.) at 25°C for 2 h. AEBSF ([4-(2-aminoethyl)-benzene-sulfonylfluoride hydrochloride], Fisher BioReagents) was added to a final concentration of 0.2 mM to stop the reaction. The mixture was applied on a gel filtration column (Sephadex G-50 medium), and washed through with PBS (pH 7.4) (10 mM sodium phosphate, 2 mM potassium phosphate, 2.7 mM potassium chloride, 137 mM sodium chloride). Purity and correct size of the protein was confirmed by Coomassie-stained SDS-PAGE and mass spectroscopy. The protein concentration was determined by measuring the absorbance at 280 nm using an extinction coefficient based on the amino acid composition.
Crystallization and structure determination
Crystals were grown using the vapor diffusion hanging-drop method; 5.2 mg/mL VpreBJ in solution was placed over a well containing 0.1 M imidazole (pH 6.5) and 1 M sodium acetate, and crystals grew in 7 d. A large, hexagonal prism-shaped crystal was cryo-protected with well solution plus 30% ethylene glycol before flash-freezing in liquid nitrogen. All data sets were collected on an Oxford diffraction sealed tube CCD diffractometer (Table 1). The data were processed with MOSFLM and CCP4 suite (Collaborative Computational Project, Number 4 1994).
The structure was solved by molecular replacement, using phase information from the homologous model 2MCG, a λ light chain. The crystallographic model was built using COOT (Emsley and Cowtan 2004), and refined using Refmac5 (Murshudov et al. 1997). Iterative rounds of model building and refinement resulted in an Rfactor of 18.3% and Rfree of 23.0%. The final model consists of residues 3–116 of VpreBJ, plus two C-terminal alanines that belong to the vector. There are 96 non-glycine, non-proline, non-terminal residues. Ramachandran analysis using PROCHECK showed that a total of 90.6% of non-glycine and non-proline residues are in the most favored regions of Ramachandran space, with 8.3% in additional allowed areas and 1.1% in disallowed regions. Residue 102, the only residue in the disallowed region, belongs to the engineered CDR3 loop, which is highly flexible. For five residues of CDR3, residues 99–103, the otherwise outstanding electron density is discontinuous.
Secondary structure was assigned using DSSP (Kabsch and Sander 1983). Figures were prepared with PyMOL. Homologs were searched for in the NCBI protein database, and sequences were aligned in CLUSTALW (Thompson et al. 1994). The structure of VpreBJ was compared with the six most homologous mature λ VL structures (accession codes: 1ADQ, 2DD8, 1CD0, 2CD0, 1NL0, 1W72), the structure used for molecular replacement (2MCG), and with the CL part of 14.1 selected VLs (accession codes: 1AQK, 2A9M, 8FAB, 1LIL, 4BJL, 1Q1J, 1JVK, 1RZF, and 2FL5). A total of three VL were randomly chosen for better graphical display in Figure 2.
Analytical ultracentrifugation
The apparent molecular mass of VpreBJ was determined by sedimentation equilibrium using a Beckman ProteomeLa XL I ultracentrifuge. Purified VpreBJ was analyzed at two different concentrations (20 and 30 μM) in PBS (pH 7.4). The protein was equilibrated at rotor speeds of 25,000 and 32,000 rpm for 24 and 30 h at 20°C. Absorbance scans at 280 nm were fit to Equations 1 and 2 describing the equilibrium sedimentation of a homogeneous single ideal species and that representing a monomer–dimer equilibrium:
 |
where Abs(r) is the absorbance at radius r, A′ is the absorbance at reference radius x 0, H = (1 − νρ)ω2/2RT, where ν is the partial specific volume, ρ is the solvent density, and ω is the angular velocity in radians/s, M is the apparent molecular weight, K d(2,1) a is the dissociation constant in absorbance units, and B is the absorbance of the blank. Data were fit using Igor Pro v5.03 and partial specific volumes and solution densities were calculated using the program SEDNTERP. The K d(2,1) a in absorbance units was converted into concentration units, K d(2,1) c, using the relation shown below with the assumption that the molar extinction coefficient of the dimer is twice that of the monomer (εm):
Data deposition
The sequence of both VpreBJ and VpreBUJ were deposited in GenBank with accession codes EU162130 and EU162131. The coordinates of VpreBJ were deposited in the Protein Data Bank, PDB ID 3BJ9.
Electronic supplemental material
The electronic supplemental material of a figure showing the superimposition of the 1H, 15N-HSQC spectra of VpreBUJ and VpreBJ, respectively, with a description of the experimental conditions is available online.
Acknowledgments
We thank Dr. Gillian Henry and Dr. James Sudmeier for their assistance with the acquisition of the NMR data. Dr. Gretchen Meinke and Dr. Paul Balbo are acknowledged for their help with X-ray crystallography. This work was supported by NIH grant AI064433.
Footnotes
Supplemental material: see www.proteinscience.org
Reprint requests to: James D. Baleja, Department of Biochemistry, Tufts University School of Medicine, 136 Harrison Avenue, Boston, MA 02111, USA; e-mail: Jim.Baleja@tufts.edu; fax: (617) 636-2409.
Article and publication are at http://www.proteinscience.org/cgi/doi/10.1110/ps.073269808.
References
- Bankovich, A.J., Raunser, S., Juo, Z.S., Walz, T., Davis, M.M., Garcia, K.C. Structural insight into pre-B cell receptor function. Science. 2007;316:291–294. doi: 10.1126/science.1139412. [DOI] [PubMed] [Google Scholar]
- Bradl, H., Wittmann, J., Milius, D., Vettermann, C., Jack, H.M. Interaction of murine precursor B cell receptor with stroma cells is controlled by the unique tail of λ 5 and stroma cell-associated heparan sulfate. J. Immunol. 2003;171:2338–2348. doi: 10.4049/jimmunol.171.5.2338. [DOI] [PubMed] [Google Scholar]
- Chothia, C., Novotny, J., Bruccoleri, R., Karplus, M. Domain association in immunoglobulin molecules. The packing of variable domains. J. Mol. Biol. 1985;186:651–663. doi: 10.1016/0022-2836(85)90137-8. [DOI] [PubMed] [Google Scholar]
- Chothia, C., Gelfand, I., Kister, A. Structural determinants in the sequences of immunoglobulin variable domain. J. Mol. Biol. 1998;278:457–479. doi: 10.1006/jmbi.1998.1653. [DOI] [PubMed] [Google Scholar]
- Collaborative Computational Project, Number 4. The CCP4 Suite: Programs for protein crystallography. Acta Cryst. D. 1994;50:760–763. doi: 10.1107/S0907444994003112. [DOI] [PubMed] [Google Scholar]
- Emsley, P., Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- Hirabayashi, Y., Lecerf, J.M., Dong, Z., Stollar, B.D. Kinetic analysis of the interactions of recombinant human VpreB and Ig V domains. J. Immunol. 1995;155:1218–1228. [PubMed] [Google Scholar]
- Jumaa, H., Hendriks, R.W., Reth, M. B cell signaling and tumorigenesis. Annu. Rev. Immunol. 2005;23:415–445. doi: 10.1146/annurev.immunol.23.021704.115606. [DOI] [PubMed] [Google Scholar]
- Kabsch, W., Sander, C. How good are predictions of protein secondary structure? FEBS Lett. 1983;155:179–182. doi: 10.1016/0014-5793(82)80597-8. [DOI] [PubMed] [Google Scholar]
- Klein, M., Kortan, C., Kells, D.I., Dorrington, K.J. Equilibrium and kinetic aspects of the interaction of isolated variable and constant domains of light chain with the Fd' fragment of immunoglobulin G. Biochemistry. 1979;18:1473–1481. doi: 10.1021/bi00575a014. [DOI] [PubMed] [Google Scholar]
- Kline, G.H., Hartwell, L., Beck-Engeser, G.B., Keyna, U., Zaharevitz, S., Klinman, N.R., Jack, H.M. Pre-B cell receptor-mediated selection of pre-B cells synthesizing functional μ heavy chains. J. Immunol. 1998;161:1608–1618. [PubMed] [Google Scholar]
- Minegishi, Y., Hendershot, L.M., Conley, M.E. Novel mechanisms control the folding and assembly of λ5/14.1 and VpreB to produce an intact surrogate light chain. Proc. Natl. Acad. Sci. 1999;96:3041–3046. doi: 10.1073/pnas.96.6.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murshudov, G.N., Vagin, A.A., Dodson, E.J. Refinement of macromolecular structures by the maximum-likelihood method. Acta Crystallogr. D Biol. Crystallogr. 1997;53:240–255. doi: 10.1107/S0907444996012255. [DOI] [PubMed] [Google Scholar]
- Novotny, J., Haber, E. Structural invariants of antigen binding: Comparison of immunoglobulin VL-VH and VL-VL domain dimers. Proc. Natl. Acad. Sci. 1985;82:4592–4596. doi: 10.1073/pnas.82.14.4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi, K., Melchers, F. The nonimmunoglobulin portion of λ5 mediates cell-autonomous pre-B cell receptor signaling. Nat. Immunol. 2003;4:849–856. doi: 10.1038/ni959. [DOI] [PubMed] [Google Scholar]
- Rolink, A.G., ten Boekel, E., Yamagami, T., Ceredig, R., Andersson, J., Melchers, F. B cell development in the mouse from early progenitors to mature B cells. Immunol. Lett. 1999;68:89–93. doi: 10.1016/s0165-2478(99)00035-8. [DOI] [PubMed] [Google Scholar]
- Rossi, B., Espeli, M., Schiff, C., Gauthier, L. Clustering of pre-B cell integrins induces galectin-1-dependent pre-B cell receptor relocalization and activation. J. Immunol. 2006;177:796–803. doi: 10.4049/jimmunol.177.2.796. [DOI] [PubMed] [Google Scholar]
- Schiffer, M., Girling, R.L., Ely, K.R., Edmundson, A.B. Structure of a λ-type Bence-Jones protein at 3.5 Å resolution. Biochemistry. 1973;12:4620–4631. doi: 10.1021/bi00747a013. [DOI] [PubMed] [Google Scholar]
- Solomon, A., Weiss, D.T., Schell, M., Ringelberg, C., Ch'ang, L.Y., Klebig, M.L. Identification and characterization of a human Vλ5 (T1) germline gene that encodes structurally unique λ light chains. Mol. Immunol. 1997;34:463–470. doi: 10.1016/s0161-5890(97)00038-2. [DOI] [PubMed] [Google Scholar]
- Thompson, J.D., Higgins, D.G., Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonegawa, S. Somatic generation of antibody diversity. Nature. 1983;302:575–581. doi: 10.1038/302575a0. [DOI] [PubMed] [Google Scholar]