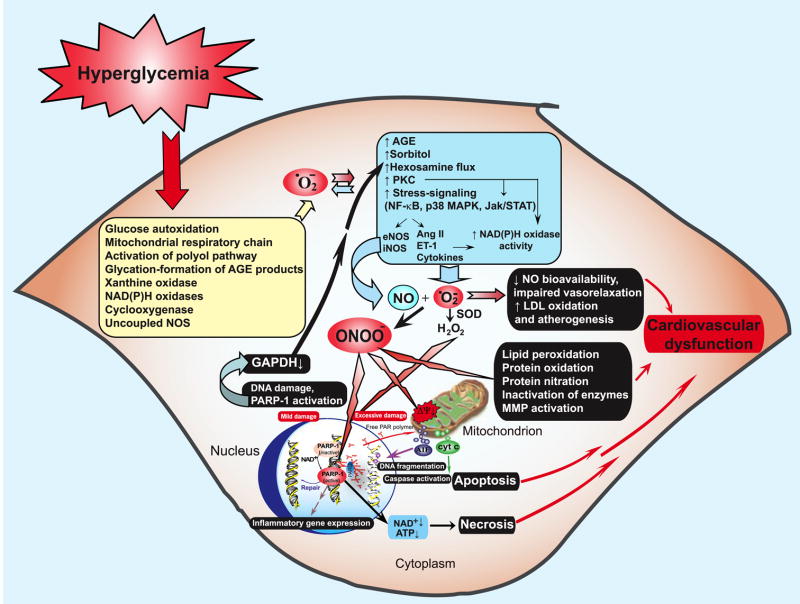
FIG. 17.
Mechanisms of cardiovascular dysfunction in diabetes: role of superoxide and peroxynitrite. Hyperglycemia induces increased superoxide anion (O2•−) production via activation of multiple pathways including xanthine and NAD(P)H oxidases, cyclooxygenase, uncoupled nitric oxide synthase (NOS), glucose autoxidation, mitochondrial respiratory chain, polyol pathway, and formation of advanced glycation end products (AGE). Superoxide activates AGE, protein kinase C (PKC), polyol (sorbitol), hexosamine, and stress-signaling pathways leading to increased expression of inflammatory cytokines, angiotensin II (Ang II), endothelin-1 (ET-1), and NAD(P)H oxidases, which in turn generate more superoxide via multiple mechanisms. Hyperglycemia-induced increased superoxide generation may also favor an increased expression of nitric oxide synthases (NOS) through the activation of NFκB, which may increase the generation of nitric oxide (NO). Superoxide anion may quench NO, thereby reducing the efficacy of a potent endothelium-derived vasodilator system. Superoxide can also be converted to hydrogen peroxide (H2O2) by superoxide dismutase (SOD) and interact with NO to form a reactive oxidant peroxynitrite (ONOO−), which induces cell damage via lipid peroxidation, inactivation of enzymes and other proteins by oxidation and nitration, and activation of matrix metalloproteinases (MMPs) among others. Peroxynitrite also acts on mitochondria [decreasing the membrane potential (Ψ)], triggering the release of proapoptotic factors such as cytochrome c (Cyt c) and apoptosis-inducing factor (AIF). These factors mediate caspase-dependent and caspase-independent apoptotic death pathways. Peroxynitrite, in concert with other oxidants (e.g., H2O2), causes strand breaks in DNA, activating the nuclear enzyme poly(ADP-ribose) polymerase-1 (PARP-1). Mild damage to DNA activates the DNA repair machinery. In contrast, once excessive oxidative and nitrosative stress-induced DNA damage occurs, overactivated PARP-1 initiates an energy-consuming cycle by transferring ADP-ribose units (small red spheres) from NAD+ to nuclear proteins, resulting in rapid depletion of the intracellular NAD+ and ATP pools, slowing the rate of glycolysis and mitochondrial respiration, and eventually leading to cellular dysfunction and death. Poly(ADP-ribose) glycohydrolase (PARG) degrades poly(ADP-ribose) (PAR) polymers, generating free PAR polymer and ADP-ribose, which may signal to the mitochondria to induce AIF release. PARP-1 activation also leads to the inhibition of cellular glyceraldehyde-3-phosphate dehydrogenase (GAPDH) activity, which in turn favors the activation of PKC, AGE, and hexosamine pathway leading to increased superoxide generation. PARP-1 also regulates the expression of a variety of inflammatory mediators, which might facilitate the progression of diabetic cardiovascular complications. [From Pacher and Szabo (996), with permission from Elsevier.]