FIG. 3.
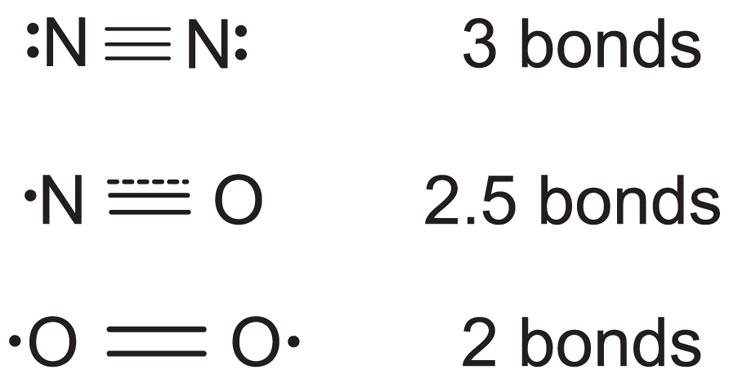
The chemical structure of nitric oxide is intermediate between molecular oxygen and nitrogen. The dot illustrates the unpaired electron on nitric oxide and two unpaired electrons on oxygen. These unpaired electrons are in antibonding orbitals, counteracting the three bonding orbitals characteristic of nitrogen gas. Thus nitric oxide has effectively 2.5 bonds and a slightly longer distance separating the nuclei. Oxygen has only two bonds and an even longer intranuclear distance.
