FIG. 6.
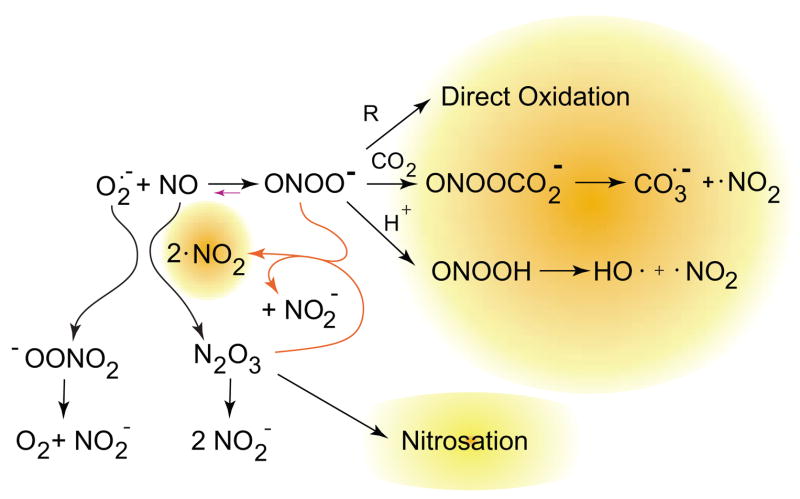
The interplay of nitric oxide, superoxide, peroxynitrite, and nitrogen dioxide. When nitric oxide and superoxide are both present, they may also react with nitrogen dioxide to form N2O3 and peroxynitrate. Peroxynitrate decomposes to give nitrite and oxygen, while N2O3 can react with thiols to give nitrosothiols or with hydroxide anion to give nitrite. Goldstein et al. (452) showed that it also reacts at a diffusion-limited rate with peroxynitrite to yield two molecules of nitrogen dioxide and one of nitrite. This creates a cycle to generate more nitrogen dioxide when bolus additions of peroxynitrite are added at neutral pH and substantially increases the number of potential reactions occurring. These same reactions will also occur in vivo, particularly when nitric oxide is produced faster than superoxide.