FIG. 8.
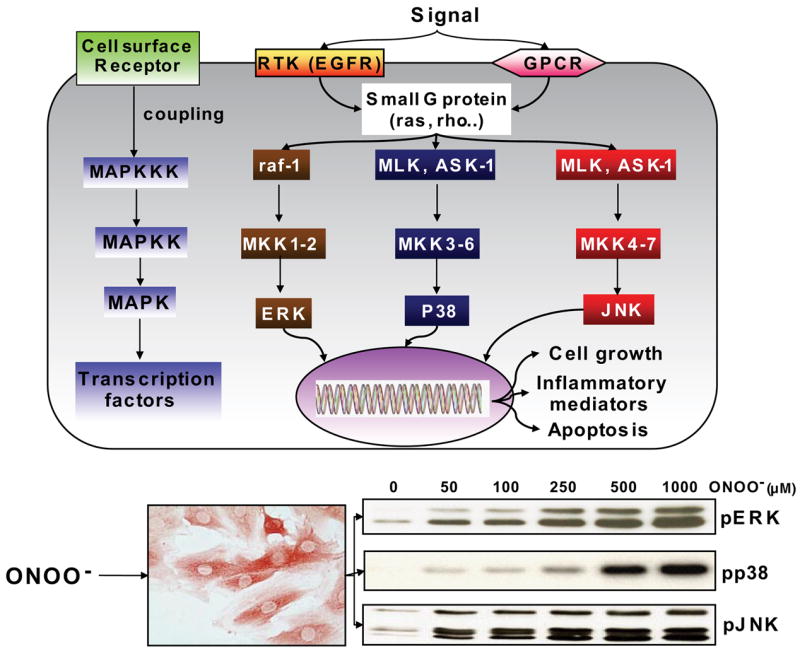
Schematic diagram of mitogen-activated protein kinase (MAPK) signaling and stimulating effects of peroxynitrite. MAPKs are activated by a dual phosphorylation at a specific tripeptide motif, as indicated on the left, mediated by a conserved protein kinase cascade, involving MAPK kinases (MAPKK or MKK) and MAPK kinase kinases (MAPKKK or MKKK). The activation of the upstream MKKK is mediated by various cell surface receptors, including G protein-coupled receptors (GPCRs) and receptor tyrosine kinases (RTK), such as the receptor for epidermal growth factor (EGFR), which activate several small G proteins, such as Ras, Rho, Rac, and Cdc42. Three groups of MAPKs exist in mammalian cells, including extracellular signal-regulated protein kinase (ERK), p38 MAPK, and the c-Jun NH2-terminal kinase (JNK), whose upstream signaling intermediates include raf-1 and MKK1–2 (ERK pathway), MLK/Ask-1 (mixed lineage kinase/apoptosis-signal regulating kinase-1) and MEK 3–6 (p38), and MLK-1/Ask-1 and MKK 4–7 (JNK). Downstream targets of MAPKs are transcription factors, enzymes, and various proteins, which regulate cell growth, apoptosis, as well as inflammation. The Western blots at the bottom show the activation pattern of the three MAPKs, evidenced by their phosphorylation, induced in the cardiomyoblast cell line H9C2 by treatment with increasing concentrations of peroxynitrite. [Adapted from Pesse et al. (1024).]
