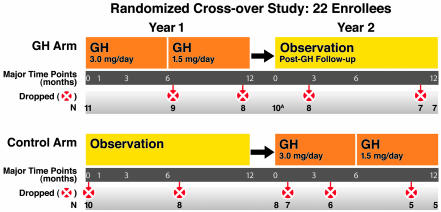
Figure 1. Study design: prospective, randomized, open-label crossover study.
The two-year study design is depicted. Eleven participants each were randomized either to receive GH for 1 year (3.0 mg GH subcutaneous injection daily for 6 months, then 1.5 mg daily for 6 months) while continuing their usual ARV (GH Arm); or to continue usual ARV for 1 year and then cross over to GH treatment and ARV in the second year (Control Arm). Unscheduled changes in GH treatment (premature dose reduction, temporary interruption, or permanent discontinuation) were made by the study investigators as indicated for management of AEs. Major time points, number of participants, and details of dropped participant data from the indicated arm are shown. Additional details of study design and data exclusion can be found in Methods. ATwo GH arm participants who terminated GH early (after month 6) were followed for 1 year after GH. These data are included in 1-year-post-GH follow-up.