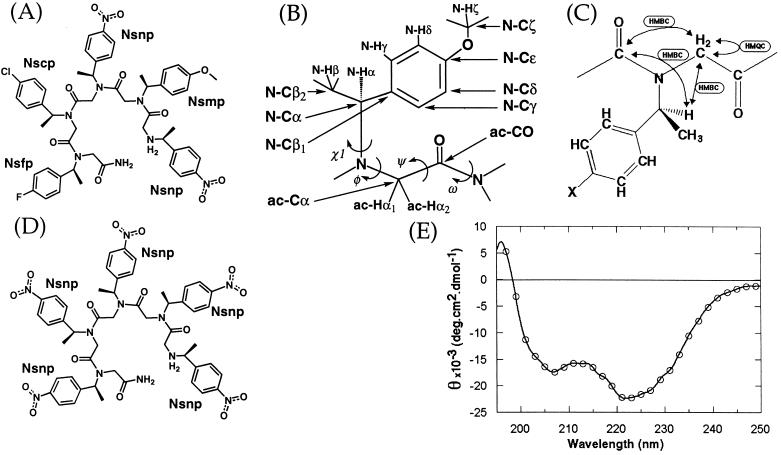
Figure 1.
(A) Compound 1 (Nsnp1-Nsmp2-Nsnp3-Nscp4-Nsfp5). Nsnp = (S)-N-(1-(p-nitrophenylethyl))glycine, Nsmp = (S)-N-(1-(p-methoxyphenylethyl))glycine, Nscp = (S)-N-(1-(p-chlorophenylethyl))glycine, Nsfp = (S)-N-(1-(p-fluoro-phenylethyl))glycine. The amide bonds are drawn in their cis configurations. (B) Atom and dihedral nomenclature. These are shown for Nsmp. The nomenclatures for the other residues are identical except for the absence of Nsmp-Cζ and Nsmp-Hζ atoms. Atom names follow (15); however, the side chain name subscript has been dropped when not referring to a particular residue (e.g., N-Cα instead of Nxxx-Cα) and has been omitted in this figure for clarity. Dihedral angles are named by analogy to those of peptides. (C) Simplified schematic representation of the assignment process for part of 1. Arrows indicate the connections that were used and the spectrum types with which they were established. (D) Pentamer of (S)-N-(1-(p-nitrophenylethyl))glycine [(Nsnp)5]. (E) CD spectrum of 1 in 100% methanol. The data were collected at 10°C and at a concentration of 0.1 mM.