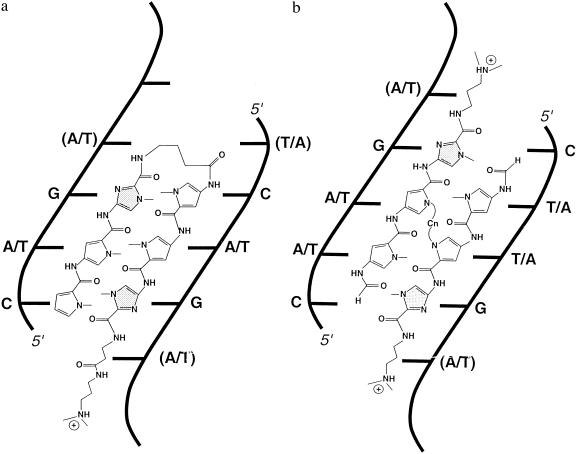
Figure 1.
Linked polyamides comprised of imidazole and pyrrole rings have been synthesized by using two methods: with a hairpin linkage (a) or stapled through a central linkage (b). When bound in the DNA minor groove, the amide groups form hydrogen bonds with the bases, positioning the heterocyclic rings directly adjacent to the edges of the bases. Hydrogen bonding and steric contacts between the rings and the bases give each ring its particular base specificity. The molecules shown here contain two imidazole rings (stippled) and four pyrrole rings (unstippled). The hairpin-linked polyamide binds to sequences of the form C-A/T-G, where A/T refers to degenerate binding to either adenine or thymine. The stapled polyamide with the same rings will bind with a shifted phasing of the rings to sequences of the form C-A/T-A/T-G. In both cases, the actual target sequence will include an additional A/T bp at each end (in parentheses), which is recognized by atoms in the tails and linkers of the polyamides.