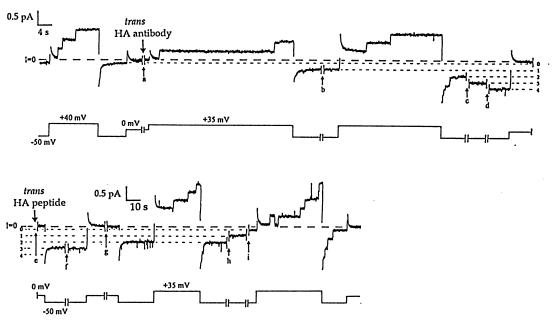
Figure 3.
The effect of trans anti-HA on HA-inserted colicin Ia at the single-channel level, and the reversal of this effect in the presence of HA peptide. For each pair of traces, the upper trace is membrane current and the lower trace is voltage. Several minutes before the start of the record, 0.5 μg of HA-inserted colicin Ia (mutant HA-II) was added to the cis compartment, and the membrane was pulsed between positive and negative voltages to confirm normal voltage gating. Near the beginning of break a (10 min), 20 μg of anti-HA was added to the trans compartment. After break a, the first pulse to +35 mV began with all channels closed, followed by the opening of two channels; when the voltage was stepped to −50 mV, instead of the rapid closing seen before antibody addition, one of the channels remained open. A second channel was locked open after break b (5 min), and a third and fourth channel were similarly locked open after channel openings during breaks c and d (2 min each). The short, dashed lines indicate the current levels at −50 mV with 0, 1, 2, 3, and 4 channels locked open. As in Fig. 2, it appears that trans anti-HA bound the translocated HA epitope in the open colicin channels and prevented the channels from closing. We attempted to reverse this effect of trans anti-HA by adding trans HA peptide to block the binding sites on the antibody. In the middle of break e (4 min.), 10 μg of HA peptide (a 68-fold molar excess) was added to the trans compartment. No additional channels were locked open over the next few hours, as the membrane was pulsed between positive and negative voltages. One of the locked-open channels closed quickly (within 2 min of peptide addition). Three channels remained open through break f (33 min), but one closed during break g (2 min) while the membrane was held at 0 mV. (Note the change in time scale after break g.) The remaining two channels closed, one at a time, during breaks h (85 min) and i (60 min). The channels subsequently showed normal voltage gating. Presumably, the locked-open colicin channels were able to close only after the antibody had dissociated from the HA epitope; the HA peptide was needed to prevent new antibody molecules from binding. Channels in the locked-open state showed greater rectification (conductance was 9 pS at +35 mV vs. 5 pS at −50 mV) than channels in the normal open state (9 pS vs. 7 pS). The solutions on both sides of the membrane were the same as in Fig. 2, with the addition of 5 mM DTT. The current transient following each voltage step was a result of the slow relaxation of the recording system at the high gain used here. The recording was taken from a chart record filtered at 10 Hz.