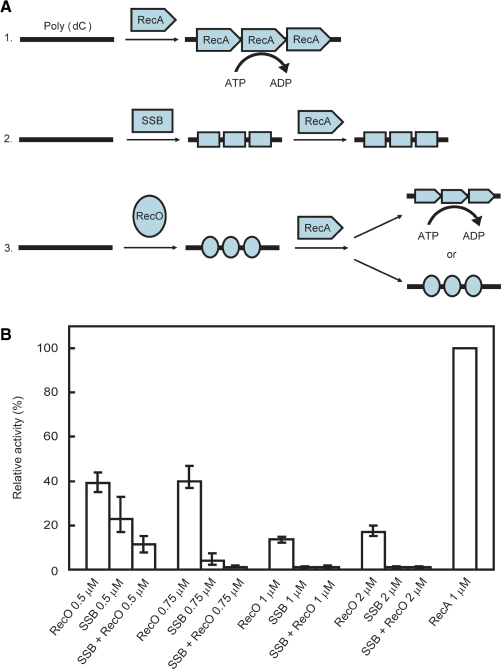
Figure 5.
The measurement of the ssDNA-dependent ATPase activity of RecA. (A) Schematic representation of the ATPase activity of RecA. 1. RecA hydrolyzes ATP in an ssDNA-dependent manner. 2. When SSB binds to the ssDNA prior to RecA, RecA ATPase activity is inhibited. 3. The effect of RecO binding to ssDNA prior to RecA on ATPase activity is not known. (B) The ssDNA-dependent ATPase activity of RecA in the presence of RecO and/or SSB. The ATPase activity of 1 μM RecA in the absence of RecO and SSB was defined as 100%. The ATPase activity of RecA in the presence of the indicated concentrations of SSB and/or RecO was measured. Each sample was incubated with a 340-mer poly (dC) prior to RecA, then 1 μM of RecA was added and the ATPase activity was measured. The data represent the average of three independent experiments.