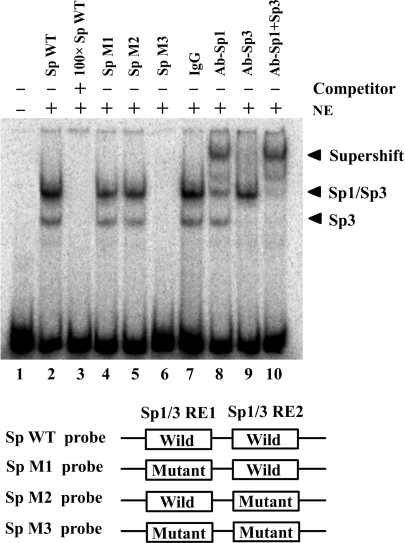
Figure 4.
Specific binding of Sp1 and Sp3 to the critical CIDE-A promoter region. The binding of Sp1 and Sp3 to the CIDE-A core promoter was determined by electrophoretic mobility shift analysis (EMSA). By using 32P-labeled 35-bp double-stranded oligonucleotides containing wild or mutated Sp1/Sp3-binding sites as probes, EMSAs were performed with the same amount of nuclear extracts (NE) from Hela cells, and the products were separated on a 4% polyacrylamide gel (lanes 2–10). Lane 1, free probe; lane 2, 32P-labeled wild-type Sp1/Sp3 consensus oligonucleotides were mixed with nuclear proteins; lane 3, the same reaction was performed as that in lane 2, except for the presence of a 100-fold excess of unlabeled wild-type Sp1/Sp3 consensus oligonucleotides as a competitor; lanes 4–6, binding assays of 32P-labeled mutant-type Sp1/Sp3 consensus oligonucleotides mixed with nuclear proteins; lanes 7–10, 1 μg each of IgG, anti-Sp1, anti-Sp3 or both anti-Sp1 and anti-Sp3 antibodies were added to the binding reaction mixtures with 32P-labeled wild-type probe.