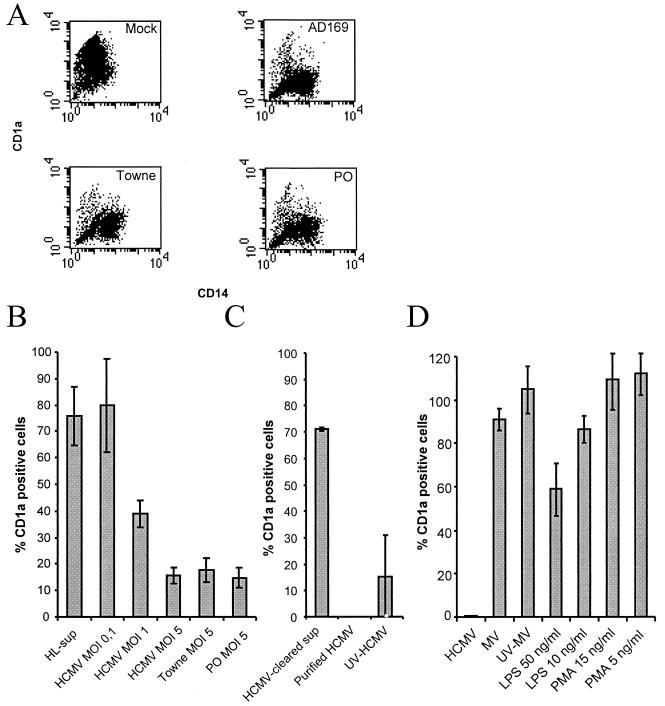
FIG. 1.
HCMV infection inhibits cytokine-induced differentiation of monocytes into dendritic cells. (A) HCMV was tested for its ability to inhibit differentiation of monocytes into dendritic cells obtained by IL-4 and GM-CSF stimulation by infecting monocytes with the laboratory strain AD169 or Towne or a clinical isolate (PO) at an MOI of 5. The figure presents a representative sample of a flow cytometric analysis of the expression of the cell surface molecules CD1a (y axes represent the mean fluorescent intensity of CD1a) and CD14 (x axes represent the mean fluorescent intensity of CD14) on mock-infected dendritic cells and HCMV-infected monocytes. (B) HCMV was tested for its ability to affect cellular differentiation by infecting cells with the laboratory strain AD169 at an MOI of 0.1 to 5, laboratory strain Towne at an MOI of 5, and a clinical isolate (PO) at an MOI of 5. Supernatants from mock-infected fibroblasts (HL-sup) were also used for infection. (C) To test whether the HCMV-induced block in cellular differentiation was dependent on soluble factors, filtered virus-free supernatants were used for infection of cells. To test if the HCMV-induced block in cellular differentiation was directly dependent on the virus particle, ultracentrifuged pure virus particles were used for infection. To test if the inhibition was dependent upon active virus replication or virus uptake by the cell, we used UV-inactivated (replication-deficient) virus for infection of cells. (D) To determine if the inability of the cells to differentiate into dendritic cells was an HCMV-specific phenomenon, monocytes were infected with measles virus or treated with phorbol myristate acetate and LPS. The percentage of CD1a-positive cells in the individual sample compared to the uninfected control was determined. The data represent mean and standard error of the mean values for six separate experiments.