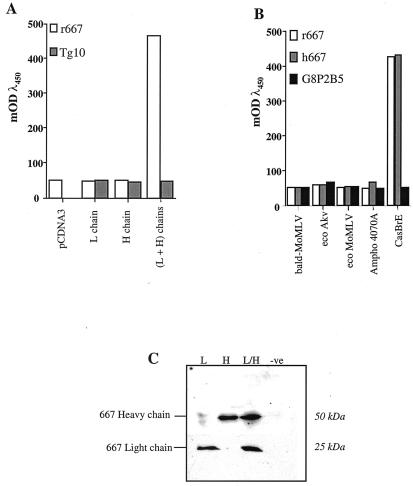
FIG. 1.
Functional characterization of r667 heavy- and light-chain cDNAs. (A) Binding of r667 heavy and light chains to CasBrE Env. Cells were transiently transfected with PM514 and PM524 expression vectors coding for r667 light and heavy chains, respectively, either individually or in combination. The void pcDNA3 vector as well as PM117 and PM124 coding for the Tg10 anti-human thyroglobulin light- and heavy-chain cDNAs, respectively, were used as negative controls. Culture supernatants were then tested by ConA ELISA using purified FrCasE particles as a source of CasBrE Env. (B) Specificity of recognition of r667. Cells were transiently cotransfected with PM514 and PM524 vectors, and cell culture supernatants were used in ConA ELISA with various sources of retroviruses to coat the ELISA plates. h667 and G8P2B5 antibodies were used as positive and negative controls, respectively. (C) Expression of r667 light and heavy chains in transfected cells. Cell culture supernatants from transiently transfected cells were processed for immunoblotting analysis, and r667 light and heavy chains were detected by using a specific anti-mouse-γ and anti-mouse-κ antibodies as described in Materials and Methods. L, light; H, heavy; eco, ecotropic; Ampho, amphotropic; −ve, negative control; mODλ450, milli-optical density units at 450 nm.