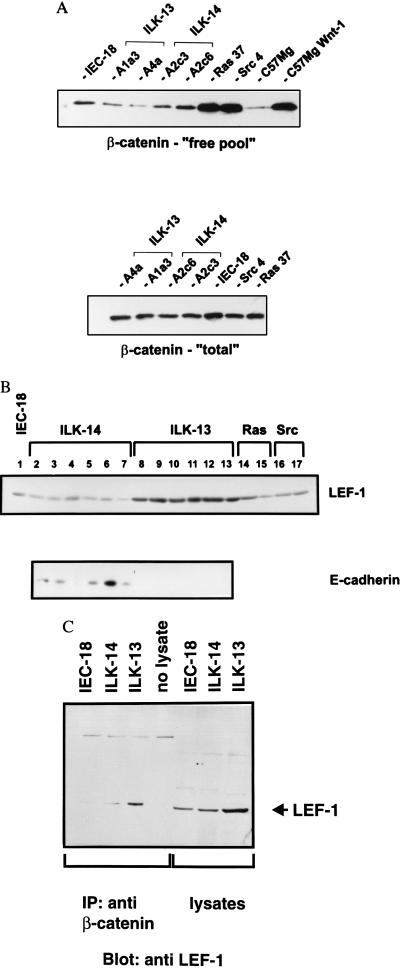
Figure 4.
(A) Immunoblot of uncomplexed and total β-catenin. Cell lysates were precipitated with Sepharose coupled to a glutathione S-transferase fusion protein containing the cytoplasmic domain of E-cadherin to bind uncomplexed pools of β-catenin (19). The pelleted beads were solubilized and electrophoresed through SDS/PAGE then Western-blotted with antibody toward β-catenin. (Lower) Comparison of the total concentrations of β-catenin. C57Mg is a mouse mammary epithelial cell line and C57Mg Wnt-1 is its counterpart stably transfected to constitutively express Wnt-1 (C57805) (32). (B) Immunoblot for LEF-1 and E-cadherin. Supernatants of cells lysed in Nonidet P-40 lysis buffer (40 μg) were electrophoresed through 8% SDS/PAGE and Western-blotted with antibody toward LEF-1 and E-cadherin. Lane 1, IEC-18; lanes 2–7, ILK-14 clones A2a3, A2c3, A2c6, A2 g3, A2 g8, and A3a1; lanes 8–13, ILK-13 clones, A1a3, A1d11, A4a, A4a3, A4c, and A4i; lanes 14 and 15, Ras clones 33, 37; and lanes 16 and 17) Src clones 2,4. (C) Coimmunoprecipitation of LEF-1 with β-catenin. Cell extracts (500 μg in Nonidet P-40 lysis buffer) were immunoprecipitated with 4 μg of β-catenin mAb and electrophoresed though 8% SDS/PAGE along with 20 μg of cell lysates alone. The gel was Western-blotted with antibody toward LEF-1.