Fig. 6.
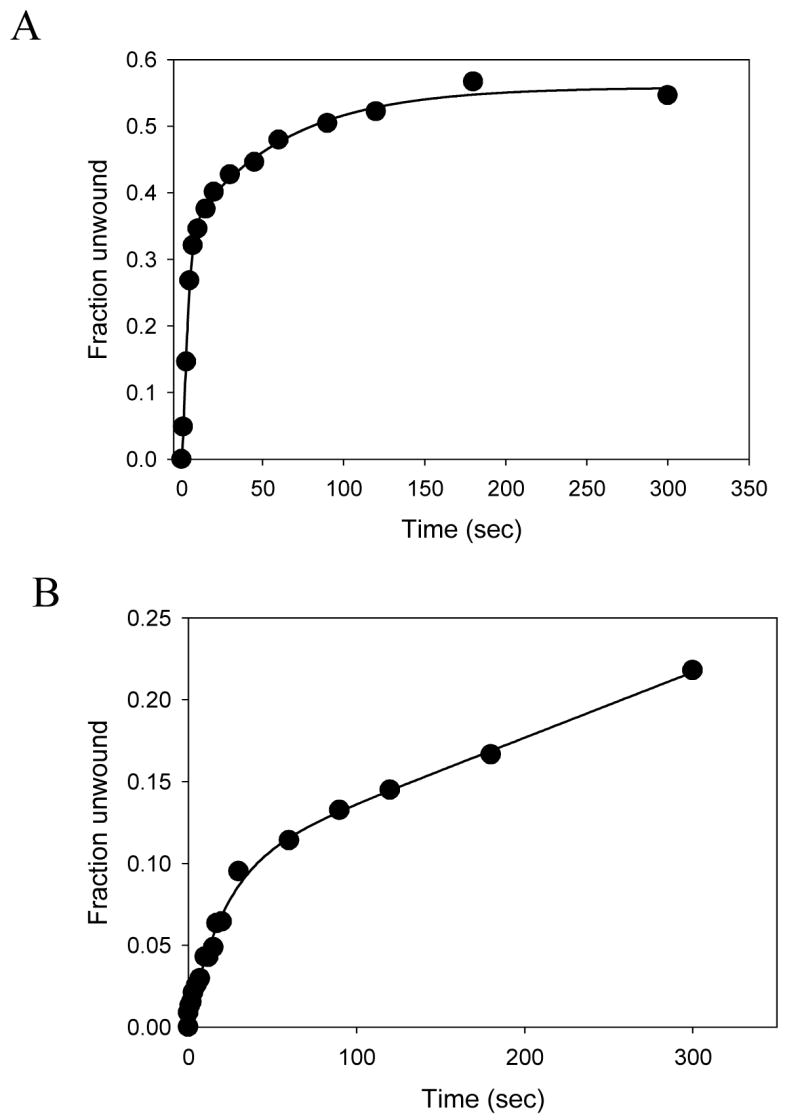
Unwinding activity of the NS3h monomer. (A) Unwinding of the 30bp substrate with a dT5 3′-loading tail and dT35 5′-tail (Table I, 5) by NS3h was carried out with in the presence of SSB and excess of the mutant NS3h. The fraction of substrate unwound is plotted as a function of time. About 45% of the substrate is unwound by NS3h at an average rate 4 bp/s. (B) Unwinding reaction was carried out using 10 nM NS3h and 10 nM 30bp forked DNA substrate. About 12% of the DNA is unwound (1.8 bp/s) in the burst phase, which is very close to the expected 16% (1/6th of the total enzyme, since the average enzyme binding site on the DNA is 7 bases).
