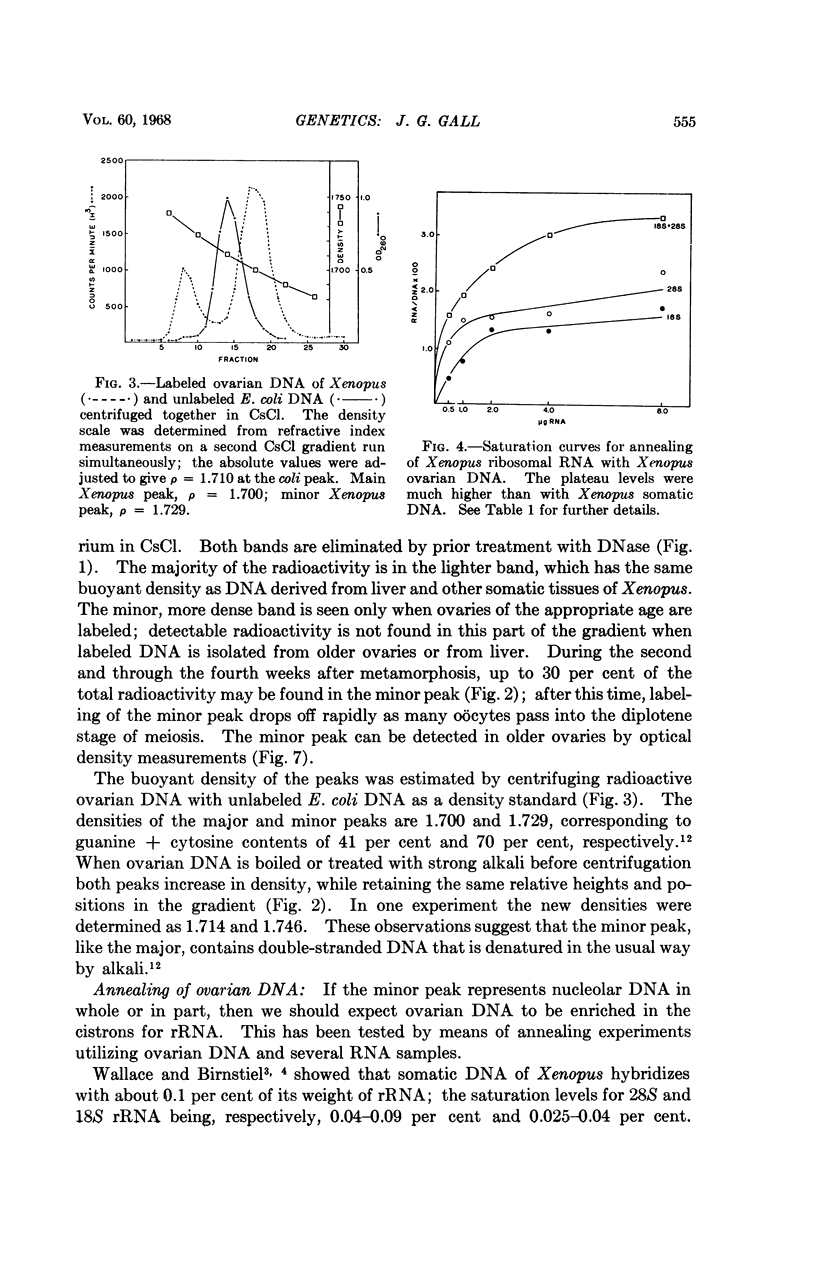
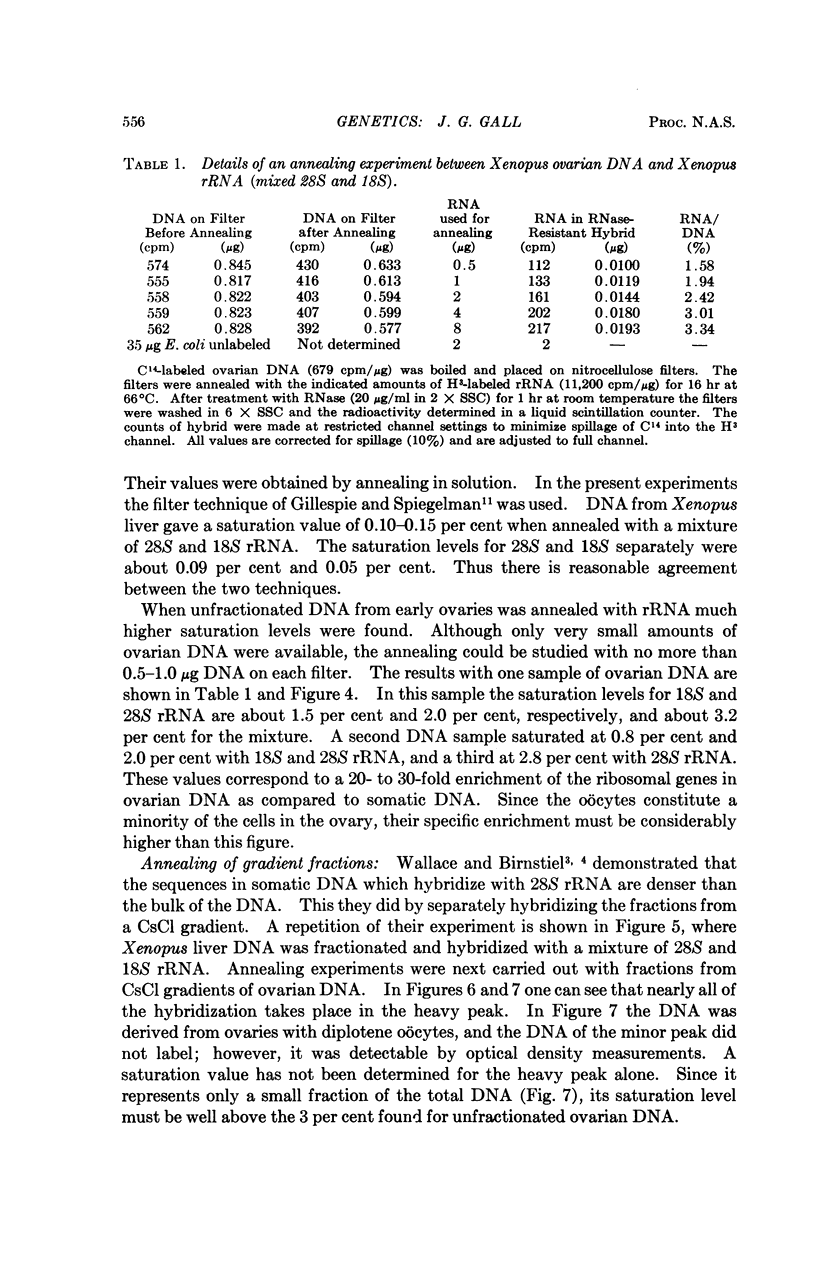
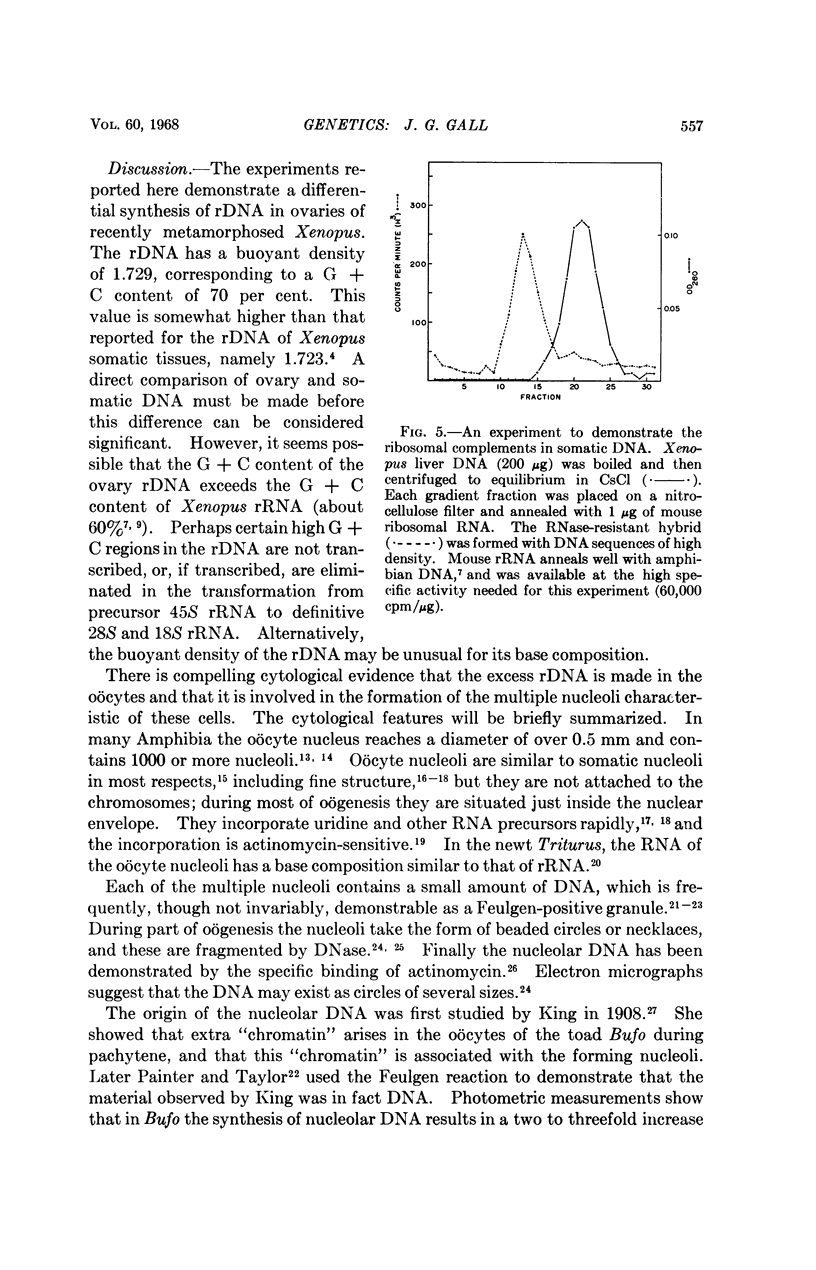
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACKLER A. W. GERM-CELL TRANSFER AND SEX RATIO IN XENOPUS LAEVIS. J Embryol Exp Morphol. 1965 Feb;13:51–61. [PubMed] [Google Scholar]
- BROWN D. D., LITTNA E. RNA SYNTHESIS DURING THE DEVELOPMENT OF XENOPUS LAEVIS, THE SOUTH AFRICAN CLAWED TOAD. J Mol Biol. 1964 May;8:669–687. doi: 10.1016/s0022-2836(64)80116-9. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Wallace H., Sirlin J. L., Fischberg M. Localization of the ribosomal DNA complements in the nucleolar organizer region of Xenopus laevis. Natl Cancer Inst Monogr. 1966 Dec;23:431–447. [PubMed] [Google Scholar]
- Brown D. D., Dawid I. B. Specific gene amplification in oocytes. Oocyte nuclei contain extrachromosomal replicas of the genes for ribosomal RNA. Science. 1968 Apr 19;160(3825):272–280. doi: 10.1126/science.160.3825.272. [DOI] [PubMed] [Google Scholar]
- Ebstein B. S. Tritiated actinomycin D as a cytochemical label for small amounts of DNA. J Cell Biol. 1967 Dec;35(3):709–713. doi: 10.1083/jcb.35.3.709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- Haggis A. J. Deoxyribonucleic acid in germinal vesicles of oocytes of Rana pipiens. Science. 1966 Nov 4;154(3749):670–671. doi: 10.1126/science.154.3749.670. [DOI] [PubMed] [Google Scholar]
- IZAWA M., ALLFREY V. G., MIRSKY A. E. COMPOSITION OF THE NUCLEUS AND CHROMOSOMES IN THE LAMPBRUSH STAGE OF THE NEWT OOECYTE. Proc Natl Acad Sci U S A. 1963 Nov;50:811–817. doi: 10.1073/pnas.50.5.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz W. Funktionsstrukturen im oocytenkern von locusta migratoria. Chromosoma. 1967;20(3):332–370. [PubMed] [Google Scholar]
- Macgregor H. C. The role of lampbrush chromosomes in the formation of nucleoli in amphibian oocytes. Q J Microsc Sci. 1965 Sep;106(3):215–228. [PubMed] [Google Scholar]
- Painter T. S., Taylor A. N. Nucleic Acid Storage in the Toad's Egg. Proc Natl Acad Sci U S A. 1942 Aug;28(8):311–317. doi: 10.1073/pnas.28.8.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peacock W. J. Chromosome replication. Natl Cancer Inst Monogr. 1965 Dec;18:101–131. [PubMed] [Google Scholar]
- RITOSSA F. M., SPIEGELMAN S. LOCALIZATION OF DNA COMPLEMENTARY TO RIBOSOMAL RNA IN THE NUCLEOLUS ORGANIZER REGION OF DROSOPHILA MELANOGASTER. Proc Natl Acad Sci U S A. 1965 Apr;53:737–745. doi: 10.1073/pnas.53.4.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritossa F. M., Atwood K. C., Lindsley D. L., Spiegelman S. On the chromosomal distribution of DNA complementary to ribosomal and soluble RNA. Natl Cancer Inst Monogr. 1966 Dec;23:449–472. [PubMed] [Google Scholar]
- SCHILDKRAUT C. L., MARMUR J., DOTY P. Determination of the base composition of deoxyribonucleic acid from its buoyant density in CsCl. J Mol Biol. 1962 Jun;4:430–443. doi: 10.1016/s0022-2836(62)80100-4. [DOI] [PubMed] [Google Scholar]
- Wallace H., Birnstiel M. L. Ribosomal cistrons and the nucleolar organizer. Biochim Biophys Acta. 1966 Feb 21;114(2):296–310. doi: 10.1016/0005-2787(66)90311-x. [DOI] [PubMed] [Google Scholar]




