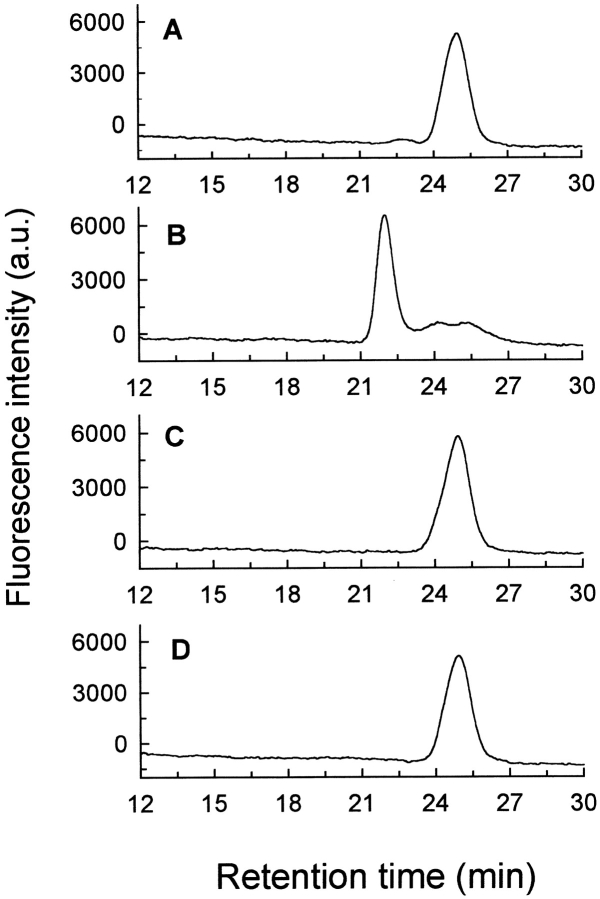
Fig. 1.
Formation of [5F]BJC–prothrombin complex and the absence of interaction of [5F]BJC with prothrombin fragments investigated by gel filtration chromatography. Superose 12 was equilibrated in TBS, and elution of [5F]BJC was monitored by the elution volume of the fluorescein fluorescence in the absence and presence of other proteins. The following samples (20-μL loop) were applied onto the column: (A) 0.33 μM [5F]BJC (0.18 μg); (B) 0.33 μM [5F]BJC (0.18 μg) + 1 μM human prothrombin (1.4 μg); (C) 0.3 μM [5F]BJC (0.18 μg) + 30 μM prothrombin fragment 1 (14 μg); (D) 0.3 μM [5F]BJC (0.18 μg) + 30 μM prothrombin fragment 2 (11 μg).