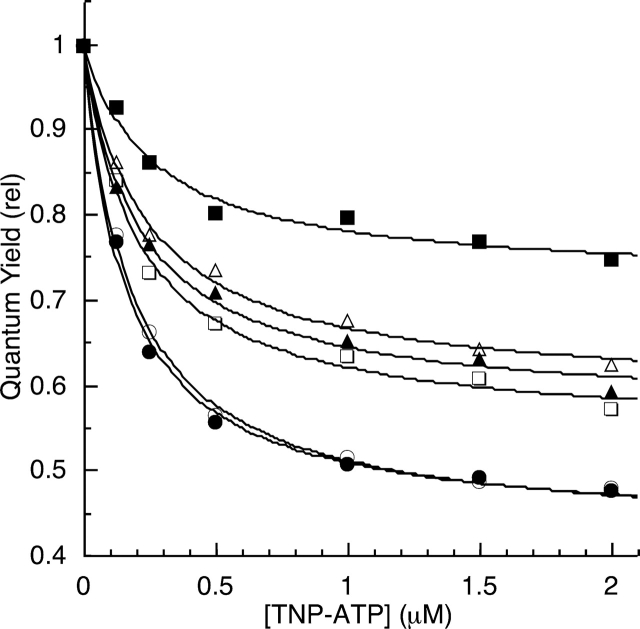
Figure 6.
Efficiency of BFP/TNP-ATP energy transfer for EGFR-ICD-BFP proteins. BFP fluorescence emission spectra of the EGFR-ICD-BFP constructs were measured in the presence of increasing TNP-ATP concentrations under phosphorylating (solid symbols) or nonphosphorylating (open symbols) conditions (see Materials and Methods). The spectra were numerically integrated to obtain relative quantum yields, which were corrected for inner filter quenching effects (see Fig. 4 ▶) and plotted. Data shown are the averages of three independent experiments. (Representative raw data are given in Fig. 3 ▶.) The titration data for EGFR-ICD-BFP (squares), EGFR-Δ1022-BFP (triangles), and EGFR-Δ976-BFP (circles) were each fit with a hyperbolic curve to estimate the energy transfer that would occur at saturation of TNP-ATP binding (Table 2). Note that the quantum yield scale is expanded.