Figure 4.
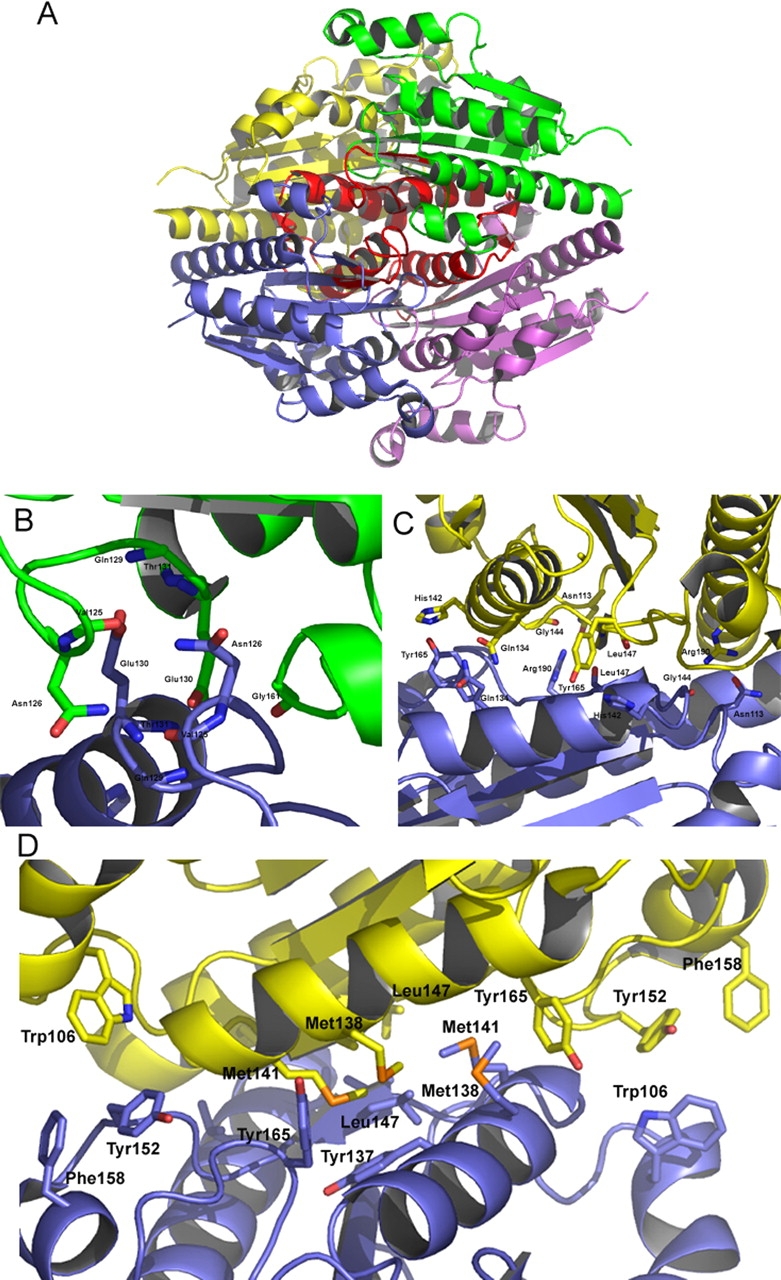
(A) A ribbon representation of WrbA from D. radiodurans highlighting the region surrounding and including α6. This region, shown in red, is located at the core of the tetramer and contributes to many of the tetramerization interactions. (B) Several residues involved in the hydrogen bond tetramerization interactions are shown. Notably, Glu130 is part of a highly conserved GGQE sequence, which interacts with the backbone amido groups of its mate’s GGQE. (C) Tyr165 interacts with His142, which contributes to properly orienting the histidine side chain to interact with FMN. Also of interest is Leu147, located on β5, which shows a two-bond anti-parallel β-sheet with its mate. (D) The hydrophobic residues contributing to the tetramerization are located along the α6 helix located at the center of the tetramer.
