Figure 3.
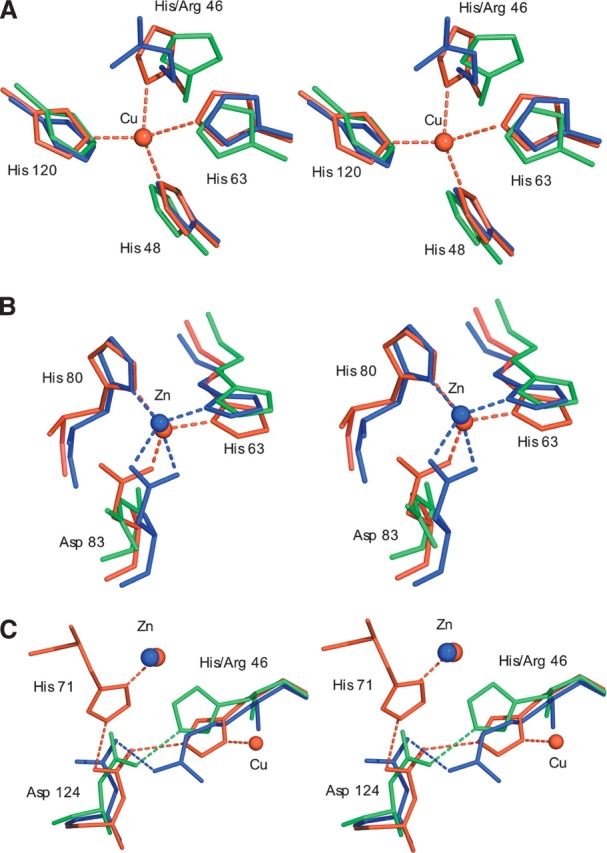
(A) Stereo figures showing the superposition of Zn-H46R (blue), wtSOD1 (red), and apo wtSOD1 (green) for the copper site. The Nη1 atom of Arg 46 occupies a position 2.8 Å from the position occupied by copper in the native hSOD structure, while the Cɛ atom resides some 2.9 Å away. The new conformation of His 120 in Zn-H46R allows the copper-ligating Nɛ2 atoms of histidines 48 and 120 to form a ~3 Å hydrogen bond. His 48 remains in a very similar conformation in the mutant and wtSOD1 structures. This preserved conformation is likely to be a consequence of the hydrogen bond between its Nδ1 atom and the O atom of Gly 61. The side chain of His 120 is shifted, occupying a position slightly closer to the vacant copper position (Nɛ2 to copper distance would be ~1.8 Å). (B) The zinc site. In Zn-H46R zinc is ligated by only three protein residues where Asp 83 acts as a bidentate ligand. His 71 is absent in Zn-H46R and is omitted from this figure for clarity, as is the water ligand observed in the Zn-H46R structure. (C) The secondary bridge. In wtSOD1, residue Asp 124 forms a hydrogen-bonded link between the copper ligand His 46 and the zinc ligand His 71. Asp 124 Oδ2 forms a strong (~2.6 Å) hydrogen bond to His46 Nɛ2, while its Oδ1 atom bonds to His71 Nɛ2. In addition, the Oδ1 atom is close to the backbone nitrogen atoms of residues 125 (3.1 Å) and 126 (2.8 Å) of the electrostatic loop, providing additional stabilization of the structure. In Zn-H46R, the mutant residue 46 maintains hydrogen bonding to Asp 124, but residue His 71 is disordered and the bridge is broken. In apo-wtSOD1, in the absence of metals His 46 hydrogen bonds to Asp 124, which adopts a different rotamer to that present in wtSOD1.
