Figure 1.
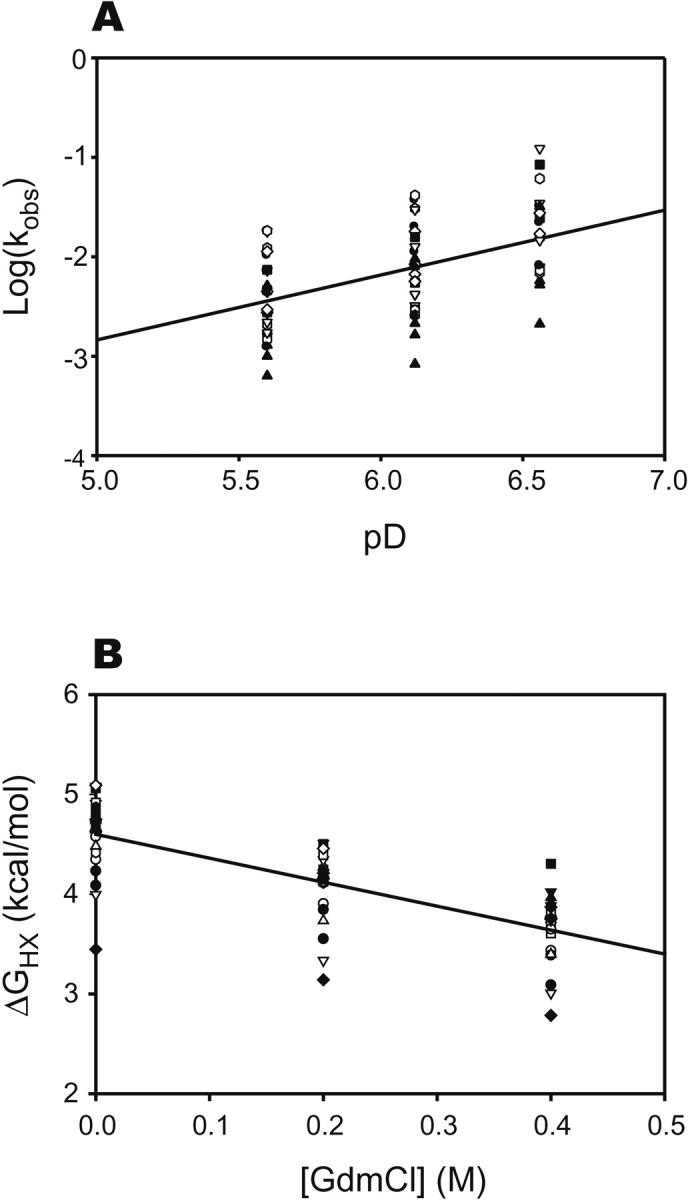
Summary of hydrogen exchange results for apo c-Src SH3. Symbols correspond to individual amides with measurable exchange rates. (A) Dependence of observed exchange rates on pD for each amide in SH3. A linear fit to all data gives a slope of 0.7. The reduced slope is due to a slight (0.2 kcal/mol) increase in the stability of SH3 over this pD range. The deviation from slope of 1 is uniform for all amides, regardless of krc, arguing against an exchange mechanism intermediate between EX1 and EX2. (B) Dependence of ΔG°opapo on GdmCl concentration. Exchange rates at most amides have a significant and a uniform denaturant dependence, suggesting that exchange proceeds through complete unfolding of the protein.
