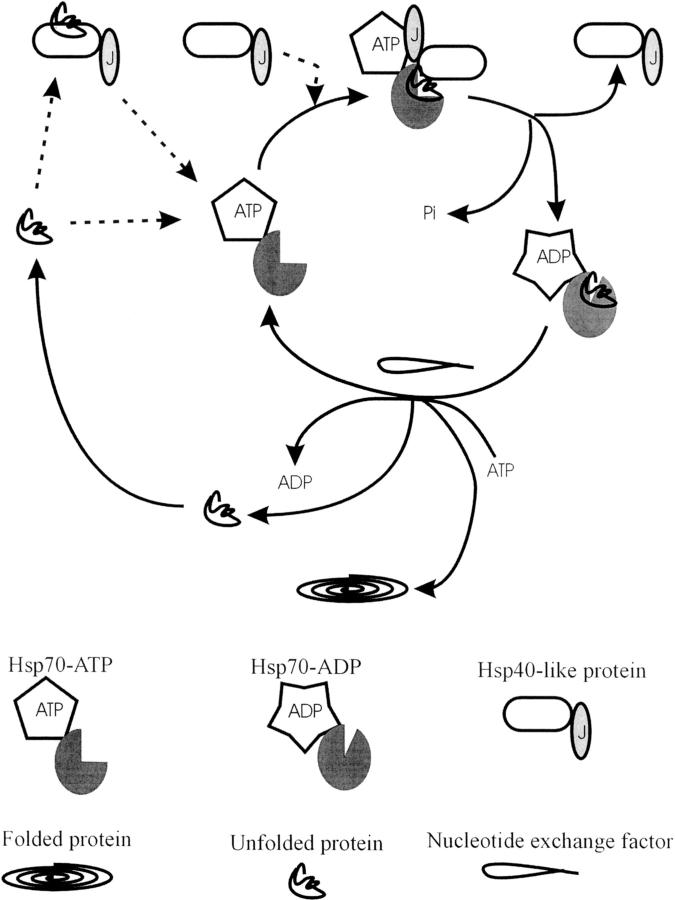
Figure 3.
Schematic showing the protein folding cycle involving the interaction of Hsp40 and Hsp40-like proteins with partner Hsp70s. Pi is inorganic phosphate. Dotted lines indicate the two different paths by which client proteins and Hsp40 or Hsp40-like proteins can enter the cycle. Client proteins are either recognized by Hsp70, following which an Hsp40 or Hsp40-like protein enters the cycle, or are presented to Hsp70 by an Hsp40 or Hsp40-like protein. ATP hydrolysis, stimulated by an Hsp40 or Hsp40-like protein, causes a conformational shift in the peptide binding domain, locking in the client protein. Nucleotide exchange then reverses the conformational shift, allowing for the release and subsequent folding of the client protein. Alternatively the client protein can re-enter the cycle.