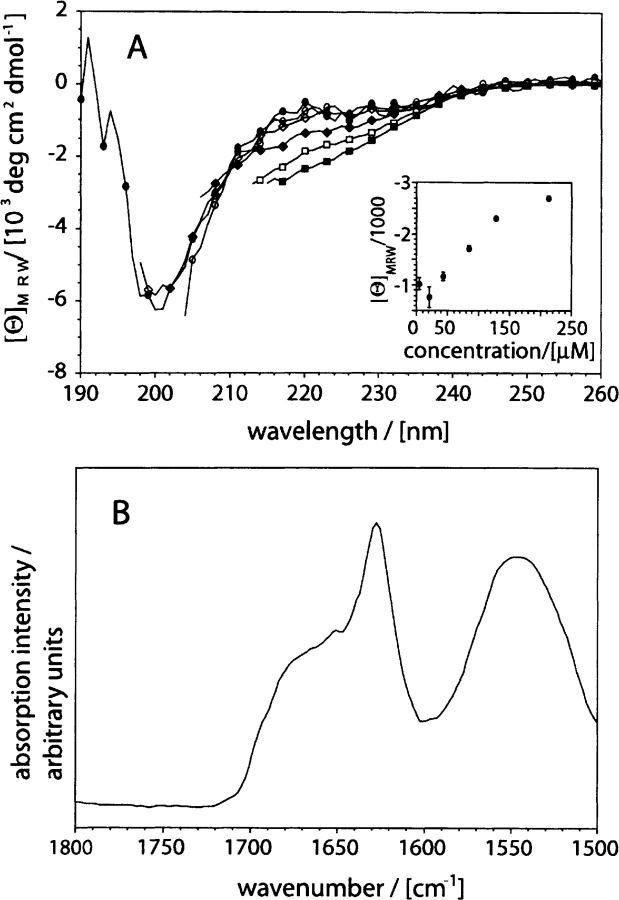
Figure 2.
Secondary structure of recombinant Aβ(1–40). (A) Far-UV CD spectra of Aβ(1–40) dissolved at different concentrations: 4.3 μM: filled circles; 21.3 μM: open circles; 42.6 μM: filled diamonds; 85.2 μM: open diamonds; 127.8 μM: filled squares; 213 μM: open squares. (Insert) Dependence of the mean residue weight ellipticity at 217 nm on peptide concentration. (B) ATR-FTIR spectrum of freshly dissolved Aβ(1–40) at 2 mg/mL concentration (457 μM). All spectra were recorded in 50 mM sodium phosphate (pH 7.4) at room temperature.