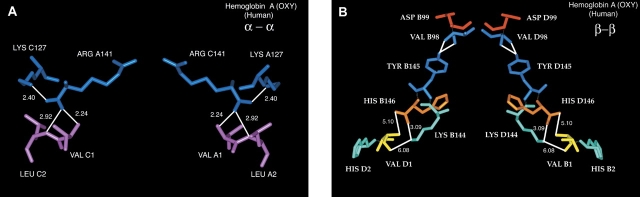
Figure 7.
N-terminal/C-terminal interactions for liganded human hemoglobin A. (A) α–α interactions; (B) β–β interactions. The coordinates used are from PDB=5 HHO by Shaanan (1983). The α-subunits in α–α are designated as chains A and C and the β-subunits in β–β are designated as chains B and D. In α–α the white lines with numbers (in angstroms) are H-bonds. The 2.24 Å distance is from one carboxylate oxygen of Arg-141 to the α-NH2 of Val-1. The 2.92 Å distance is from the same carboxylate oxygen of Arg-141 to the peptide bond N between Val-1 and Leu-2. The 2.40 Å distance is between the other carboxylate oxygen of Arg-141 to the ɛ-NH2 of Lys-127. In β–β the white lines from Val-98 are H-bonds between the peptide bond N and O of Val-98 to the phenolic OH of Tyr-145, whereas those between Val-1, His-2, Lys-144, and His-146 are distances. The 3.09 Å distance is between one carboxylate oxygen of His-146 and the ɛ-NH2 of Lys-144. The 5.10 Å distance is between the other carboxylate oxygen of His-146 and the α-NH2 of Val-1. The 6.08 Å distance is between the ɛ-NH2 of Lys-144 and the peptide bond O between Val-1 and His-2. The program Insight II (Accelrys) was used to construct these figures from the data of Shaanan (1983).