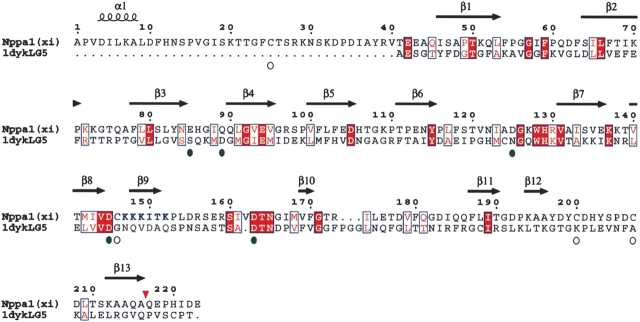
Figure 2.
Alignment of 1dyk and Npp α1(XI) collagen. The laminin α2 LG chain was used as template for 183 amino acids of Npp α1(XI) collagen. Open circles indicate the position of four cysteine residues of Npp α1(XI) collagen; green circles indicate the position of residues of potential calcium binding sites. Conserved amino acid residues are shown as white letters on red background, while similar amino acid residues are shown as red letters on white background. Positions of predicted β-strands are indicated by arrows above the sequence. Position of predicted α-helix is indicated by a coil symbol above the sequence. Amino acids are numbered from 1 to 223, with 1 defined as the first amino acid after the signal peptide cleavage site. Bone morphogenetic protein-1 proteolytic processing site is indicated by an inverted red triangle. Putative heparin binding site is indicated by blue lettering at positions 147–152.