Figure 1.
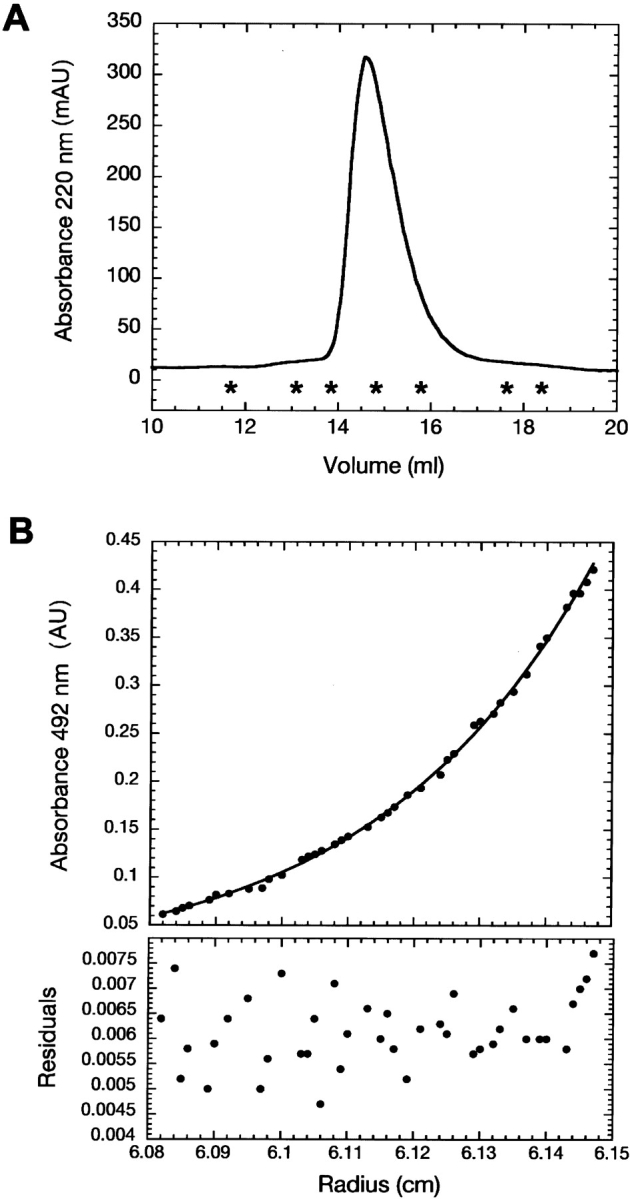
Hydrodynamic characterization of Securin. (A) Size exclusion chromatography elution profile of Securin monitored at 220 nm. Asterisks denote the positions of the molecular weight standards. (Left to right) Ferritin (440 kDa); catalase (232 kDa); aldolase (158 kDa); albumin (67 kDa); ovoalbumin (43 kDa); chymotrypsinogen (25 kDa); ribonuclease (13.7 kDa). (B) Equilibrium sedimentation experiments of fluorescein-labeled Securin at 10°C. Residuals correspond to the fitting of the data to a single exponential model to deduce the apparent molecular weight.
