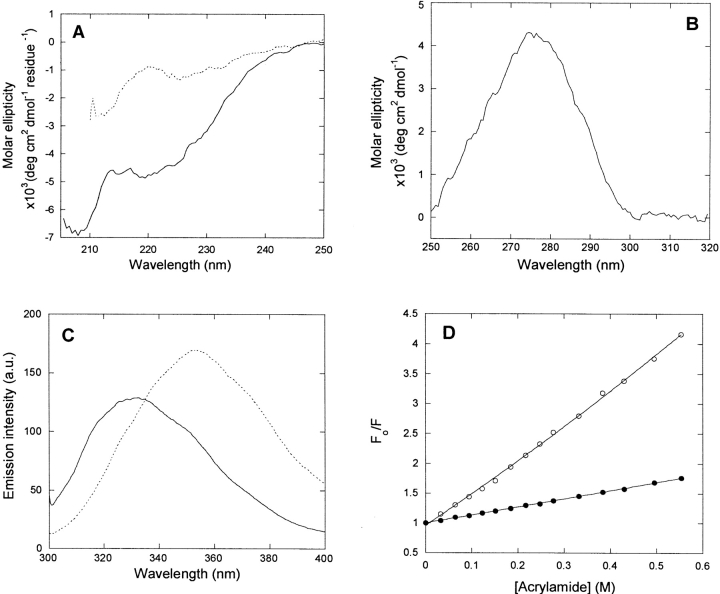
Figure 2.
Spectral properties of p25α. (A) Far-UV CD spectrum of native (solid line) and denatured (stippled line [5 M urea]) p25α. Native p25α is predicted to have 15% α-helix, 30% β-sheet, and 55% random coil, although the fit to the predicted spectrum is not good (data not shown). (B) Near-UV CD spectrum of native p25α. The peak at 280 nm indicates that the Trp residue experiences a well defined asymmetrical environment, typical of the native state. (C) Fluorescence emission spectra of native (solid line) and denatured (stippled line [5 M urea]) p25α. (D) Stern-Volmer plot of the quenching of native (•) and denatured (○ [5 M urea]) p25α. The slopes of the plots are 1.14 and 4.30 M−1, respectively, indicating that the Trp is much more accessible to acrylamide in the denatured rather than the native state.