Figure 4.
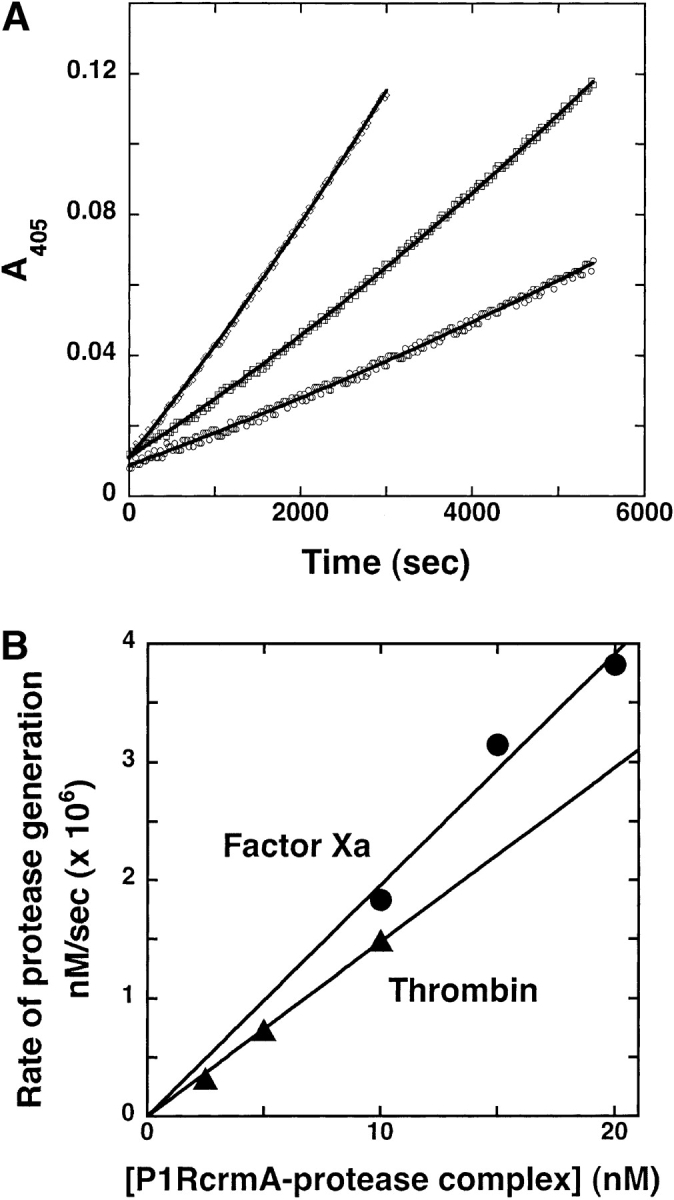
Kinetics of dissociation of P1 Arg crmA–thrombin complex. (A) P1 Arg crmA (12 μM) was mixed with 2 μM thrombin and incubated for 1 h at 25°C to form a complex. The complex was then diluted into 200 μM S-2238 substrate preincubated at 37°C to yield concentrations of 2.5 (○), 5 (□), and 10 nM (▵). Regeneration of protease from the complex was continuously monitored from the parabolic increase in absorbance at 405 nm. Solid lines are fits to the parabolic equation given in Materials and Methods. (B) Secondary plots of the fitted Δv/Δt parameter of the parabolic equation (converted to the rate of protease generation using the measured turnover number for substrate hydrolysis) are shown as a function of the complex concentration for both thrombin and factor Xa complexes, from which the first-order complex dissociation rate constant was determined.
