Figure 1.
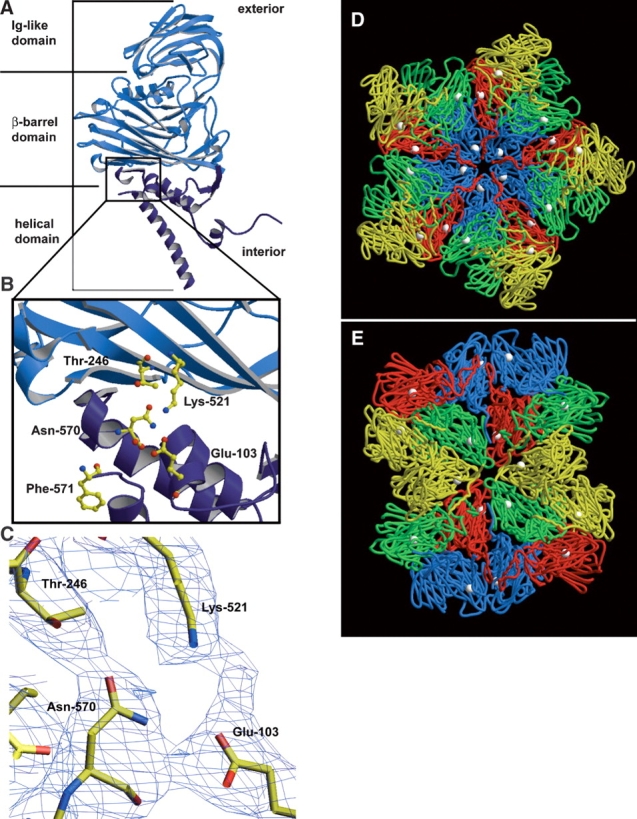
The crystal structure of NωV reveals that the cleavage site is located on the interior of the capsid in the helical region, near the β-barrel domain of the coat protein subunit. The helical region is colored dark blue in A and B. (A) A ribbon diagram of the X-ray structure of the NωV A subunit. The NωV capsid is composed of 60 copies each of A, B, C, and D subunits, which are from the same gene and related by quasi-equivalence. (B) Magnified view of the cleavage site of the NωV coat protein with ball-and-stick representations of residues believed to be involved in the autocatalytic cleavage that occurs between Asn-570 and Phe-571. (C) Electron density of intrasubunit residues near the cleavage site of NωV contoured at 0.8 σ. (D) The crystal structure of NωV capsid at the icosahedral fivefold axis of symmetry and (E) the icosahedral twofold axis of symmetry from the inside of the capsid looking out. A subunits are displayed in blue, B subunits in red, C subunits in green, D subunits in yellow, and asparagine-570 of each coat protein subunit as white spheres in D and E. The ordered N termini of neighboring subunits are approximately 10–14 Å from carbonyl carbons of Asn-570. A, B, D, and E were made with Molscript (Kraulis 1991) and Raster3D (Merritt and Murphy 1994). C was made with the program O (Jones et al. 1991).
