Figure 6.
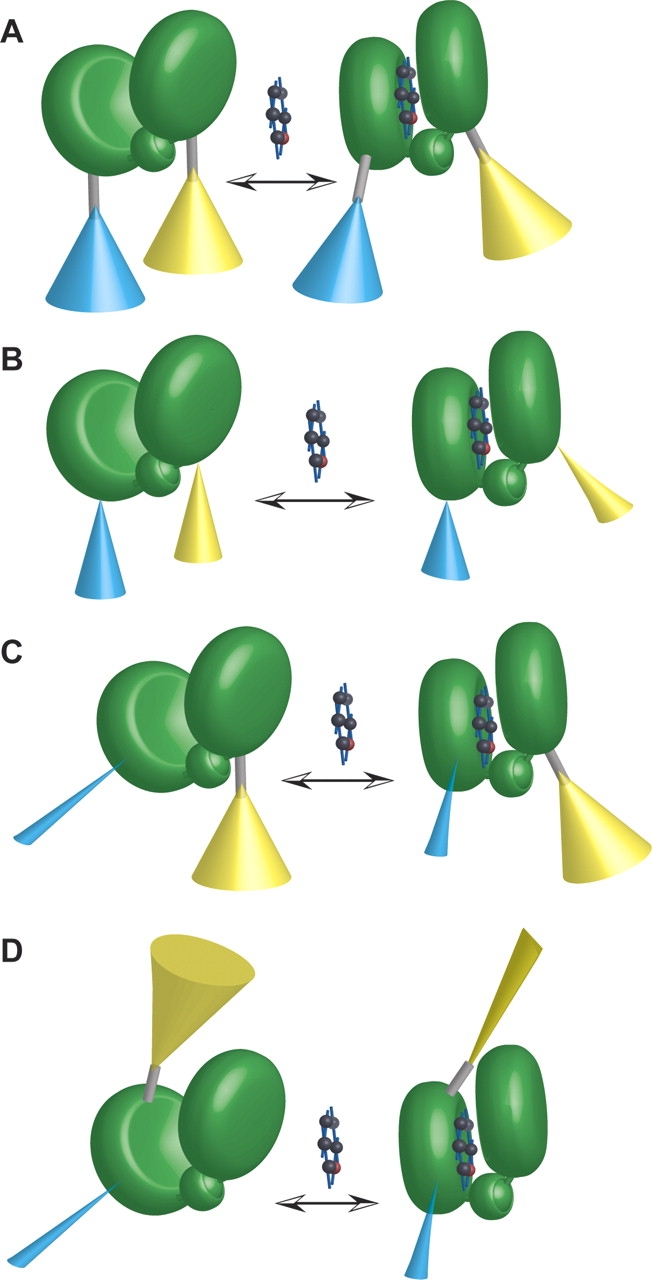
Hypothetical models of sensor function. (A) First-generation FLIPglu glucose sensor model. Fluorophores (ECFP [cyan], EYFP [yellow]) attached via flexible linkers (gray rod) to the recognition element (MglB protein, consisting of two lobes connected by a hinge [green]). The ligand (glucose) is indicated by a space-filling model. Cones represent a simplified model of the hypothetical ensemble space of fluorophore dipole. Cones are depicted comparatively wide to indicate large freedom of rotation around peptide bonds in the linkers. Thus FRET changes are expected to be relatively small. (B) Sensors with truncated linkers. Truncation reduces rotational freedom as indicated by smaller cones (reduced rotation will probably also lead to distortion of cones, not depicted). Thus FRET changes are expected to be larger. (C) Fluorophore insertions. ECFP (cyan) insertion into the recognition element leads to a dramatic reduction in rotational freedom. EYFP (yellow) is attached to the respective other lobe. Thus FRET changes are expected to be larger. (D) Fluorophore insertions. ECFP (cyan) insertion into the recognition element leads to a dramatic reduction in rotational freedom. EYFP (yellow) is attached to the same lobe carrying the insertion. A scenario is depicted in which the conformational change in the recognition element upon ligand binding restricts the rotational freedom of EYFP, providing one potential mechanism for the observation of ratio changes in the case of reporter attachment on the same lobe.
