Figure 1.
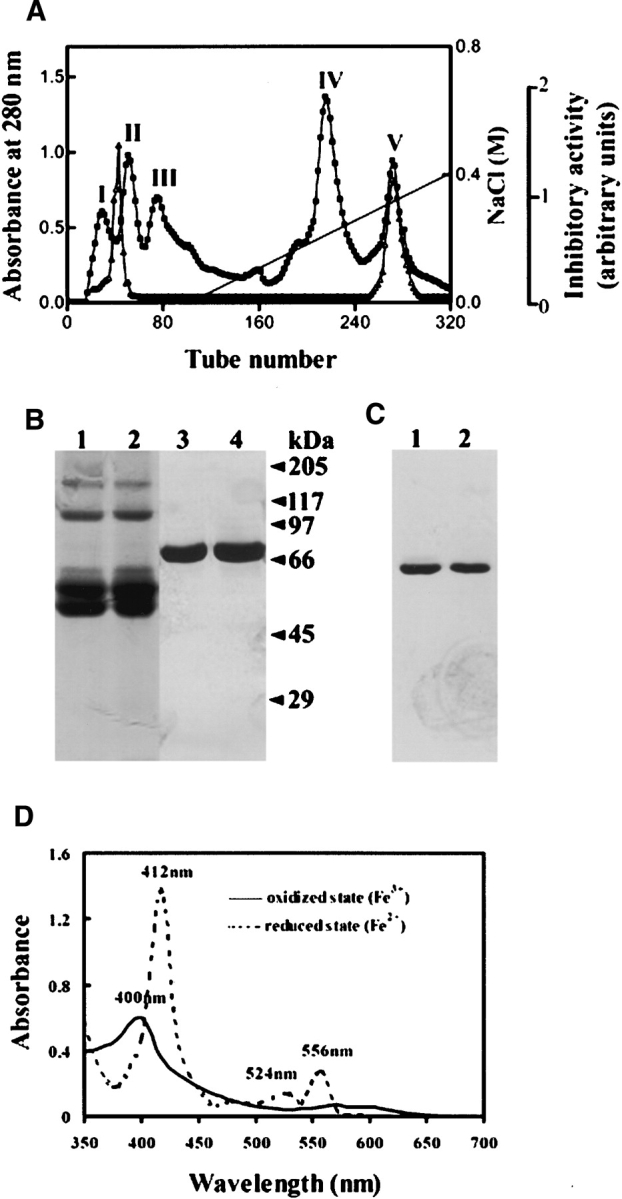
(A) Ion exchange chromatography of B. maxima skin homogenate on a DEAE Sephadex A-50 (Pharmacia) column (2.6 × 50 cm) equilibrated with 50 mM Tris-HCl buffer (pH 7.8), containing 5 mM EDTA. The elution was performed at a flow rate of 20 mL/h with a linear NaCl gradient, collecting fractions of 5 mL per tube. The protein concentration was estimated from the absorbance at 280 nm (▪). Trypsin inhibitory activity (▵) was determined as described in Materials and Methods. (B) SDS-PAGE (10% acrylamide) analysis of purified B. maxima albumin: BmA-skin (lanes 1,3), BmA-serum (lanes 2,4), non-reducing conditions (lanes 1,2), and reducing conditions (lanes 3,4). (C) PAGE in native conditions (pH 8.5) (10% acrylamide): BmA-skin (lane 1) and BmA-serum (lane 2). In both B and C, proteins were silver-stained. (D) Absorption spectra of BmA-skin in oxidized and reduced states.
