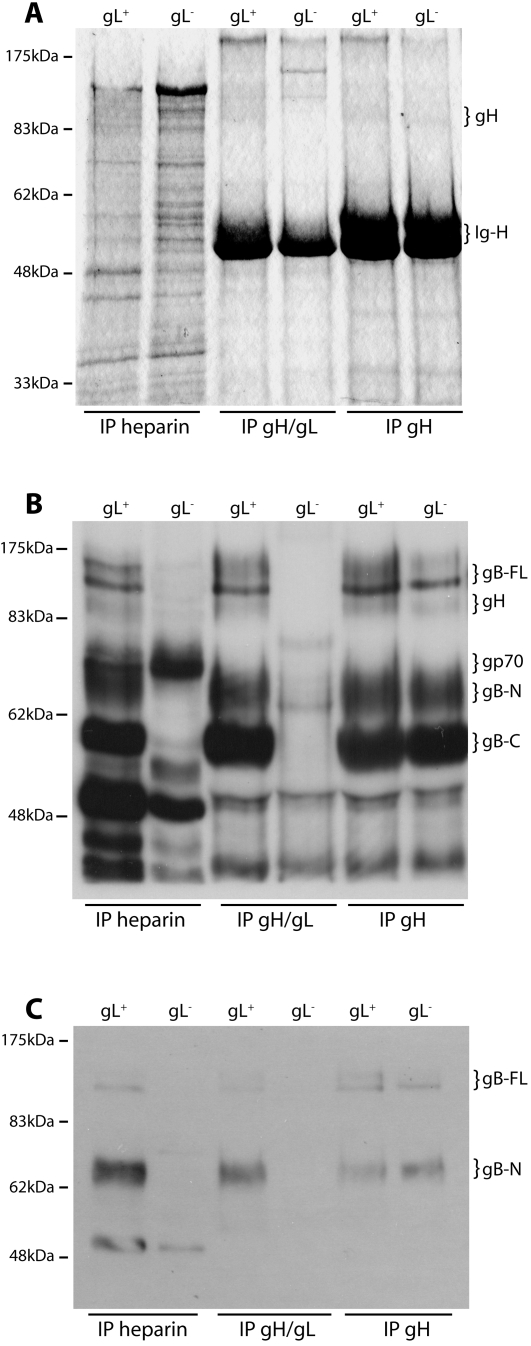
Figure 3. Virion gH/gL binds to GAGs.
A. Wild-type (gL+) and gL-deficient (gL−) virions were lysed in 1% digitonin, then immunoprecipitated with either heparin-agarose or protein A-sepharose plus mAb 7E5 (gH/gL-specific) or 8C1 (which recognizes all forms of gH). The washed precpitates were denatured, resolved by SDS-PAGE and visualized by Coomassie staining. The positions of gH (85 kDa) and IgG heavy chains (Ig-H, 50–55 kDa) are shown. The gH signal was weak but clearly visible by comparison with the gL− virion/gH/gL IP control. The data are from 1 of 2 equivalent experiments. B. The same precipitates as in A were immunoblotted with a MuHV-4-immune rabbit serum, which recognizes (among other virion proteins) gp70, gp150, gB and gH. The MuHV-4 virion glycoproteins are readily distinguished by SDS-PAGE. The positions of gH (85 kDa), gp70, full-length gB (gB-FL, 120 kDa) and the N- and C-terminal gB cleavage products (gB-N, 65 kDa; gB-C, 55 kDa) are shown. The data are from 1 of 2 equivalent experiments. C. The same precipitates were immunoblotted with mAb MG-10C11, which recognizes an epitope in gB-N. The gB-FL bands are faint because most virion gB is cleaved [18]. The identity of the 50 kDa band in the heparin IP lanes is unclear. The only glycoprotein target of MG-10C11 is gB, but it is known to also recognize a 50 kDa virion protein on immunoblots [21].