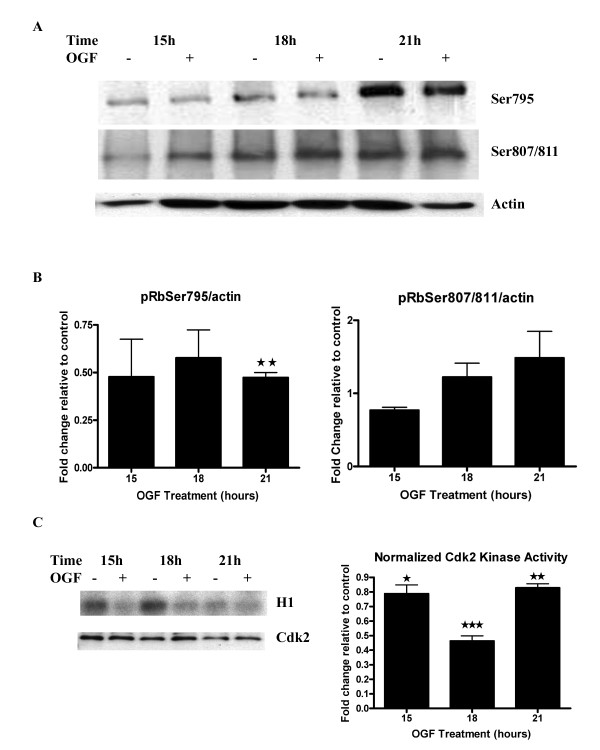
Figure 2.
Inhibition of Rb phosphorylation and Cdk2 kinase activity by OGF. (A) Synchronized BxPC-3 cells were grown in the presence of OGF or sterile water and harvested at 15, 18 and 21 hours. Equal amounts of protein were analyzed by Western blot using specific antibodies recognizing phosphorylated Rb (Ser807/811 or Ser795), and normalized to actin. (B) Densitometric analysis of Western blots in (A). Rb phosphorylation after OGF treatment is expressed relative to controls at the respective time point. Cdk2 activity as detected by phosphorylation on Rb Ser795 antibodies was reduced approximately 2-fold in OGF-treated groups relative to controls at all time points, and was significantly repressed at 21 hours (**p < 0.01). The lower level of phosphorylation at early time points resulted in higher variability due to quantifying low signals; as a consequence, the early time points are not statistically significant, but nevertheless the trend is clearly similar. No significant change in Rb phorphorylation related to Cdk4 kinase activity as assessed by phosphorylation at SER807/811 was recorded at any time point. Absolute changes in phosphorylation were less than 48%. (C) Cdk2 kinase activity was evaluated in synchronized BxPC-3 cells treated with OGF for 15, 18 or 21 hours. Cdk2 kinase activity was measured as the capacity of phosphorylation of H1 protein in the presence of radioactive ATP. Densitometric analysis of the kinase assay was performed, and the Cdk2 activity was measured relative to controls. Values represent means ± SE for 3 independent experiments. Kinase activity values from OGF-treated cultures were significantly reduced from control cells at each respective time point (*p < 0.05, **p < 0.01, ***p < 0.001).