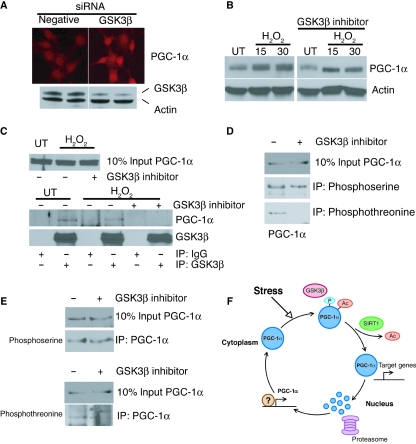
Fig. 4.
GSK3β-dependent phosphorylation of peroxisome proliferator-activated receptor-γ co-activator 1α (PGC-1α) in reponse to stress. (A) Immunofluorescent detection of PGC-1α in cells 24 h following siRNA with negative control or GSK3β-specific oligonucleotides. Knock down of GSK3β detected by Western blot (lower panel). (B) Western blot detection of PGC-1α in extracts taken at the indicated times in minutes following hydrogen peroxide treatment (350 µm); cells were grown under normal conditions or in the presence of GSK3β inhibitor VII (20 µm) prior to and during stress. (C) Detection of PGC-1α by Western blot of GSK3β immunoprecipitates from untreated or hydrogen-peroxide-treated cells (350 µm, 15 min); cells were grown in the absence or presence of GSK3β inhibitor VII (20 µm). (D) Detection of PGC-1α in phosphoserine and phosphothreonine immunoprecipitates from hydrogen-peroxide-treated cells (350 µm, 15 min); cells were grown in the absence or presence of GSK3β inhibitor VII (20 µm). (E) Detection of phosphoserine and phosphothreonine phosphorylated species by Western blot of PGC-1α immunoprecipitates from hydrogen-peroxide-treated cells (350 µm, 15 min); cells were grown in the absence or presence of GSK3β inhibitor VII (20 µm). (F) Model of PGC-1α activation in response to oxidative stress.