Figure 6.
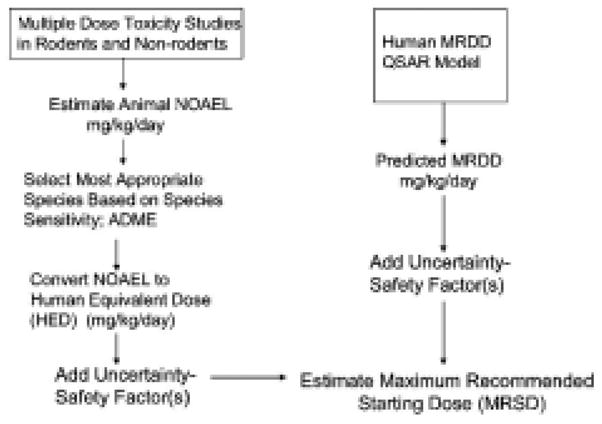
Traditional and computational approaches to selection of the Maximum Recommended Starting Dose (MRSD) for Phase 1 clinical trials.
Figure was reprinted from Regulatory Toxicology and Pharmacology 40: 185–206 (2004), “Estimating the safe and staring dose in phase I clinical trials and no observed effect level based on QSAR modeling of the human maximum recommended daily dose”, by J.F. Contrera, E.J. Matthews, N.L. Kruhlak, and R. D. Benz [79], with permission from Elsevier.
