Figure 3.
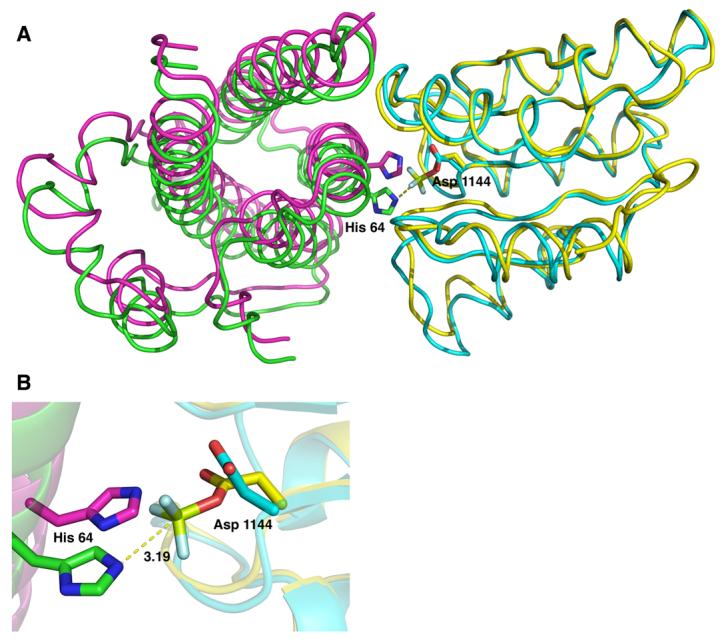
Superposition of the YPD1/SLN1-R1•Mg2+•BeF3− quaternary complex (YPD1 in green and SLN1-R1 in yellow) and the YPD1/SLN1-R1 apo complex (in magenta and cyan, respectively). A) Top view of the overlayed structures in which the αA helix of YPD1 was removed for clarity. BeF3− is shown in light green and light blue. The alignment was performed using the first 50 residues of SLN1-R1 because there is little difference in these residues between the two complexes. The rigid body shift in YPD1 from the apo complex (magenta) to the BeF3−-bound complex (green) is approximately 2.2 Å and allows for the alignment of the active site residues for phosphotransfer. B) Close-up view of the superimposed complexes showing the active site residues His 64 from YPD1 and Asp 1144 from SLN1-R1. The distance from the His 64 Nε2 atom to the beryllium atom (light green) is 3.19 Å.
