Figure 3.
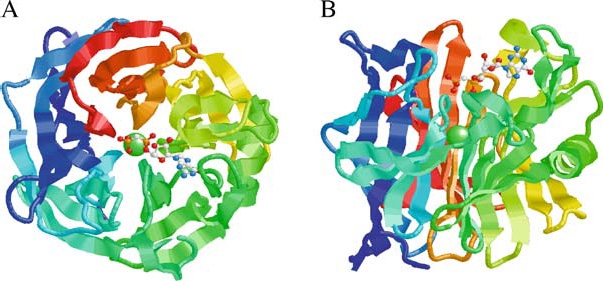
Structure of the human calcium activated nucleotidase in complex with the GMP-CP substrate analog. Ribbon diagrams of the human protein β-propeller structure as viewed along (A) or perpendicular to (B) the central tunnel. The polypeptide is colored from blue at the C-terminus through to red at the C-terminus. The structure reveals an unusual five-bladed β-propeller with five twisted β-sheets, each formed from four antiparallel β-strands. The interface between neighboring blades is predominantly hydrophobic, with residues on the adjacent faces of the β-sheets in van der Waals contact. The Ca2+ ion (green sphere) is located at the middle of the central tunnel. The GMP-CP molecule is shown in a ball-and-stick representation (CPK color scheme). The propeller has an approximate diameter of 44 Å and height of 30 Å. The atomic coordinates are available at the Protein Data Bank (PDB code 1S1D). Reprinted from Cell, Vol. 116, Dai et al., Structure and Protein Design of a Human Platelet Function Inhibitor, pp 649–659, Copyright (2004), with permission from Elsevier.
