Figure 4.
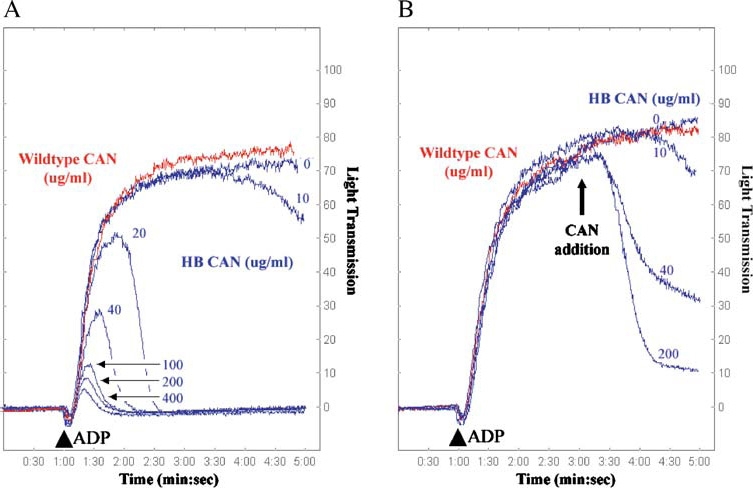
Inhibition and reversal of ADP-induced platelet aggregation using wild-type and HB (human/bedbug) mutant CAN. Platelet aggregometry and disaggregation studies were performed using human platelet-rich plasma treated with buffer (control), wild-type human CAN, or mutant G160S/L161S/K163M/T168K/E178M (denoted HB CAN). (A) In the presence of buffer (control, blue line, 0 µg/ml) platelets are strongly aggregated in response to ADP (10 µM final concentration added at 1 min, indicated by an arrowhead). HB CAN (blue traces) abrogates the ADP-induced platelet aggregation in a concentration-dependent manner, with complete blockade of aggregation at 20 µg/ml enzyme. However, wild-type human CAN (red trace) lacks the ability to prevent platelet aggregation even at a concentration of 400 µg/ml. (B) HB CAN was also tested for its ability to disrupt platelet aggregates. Addition of 10 µM ADP (arrowhead) to platelet-rich human plasma resulted in maximal platelet aggregation within the first 4 min. HB CAN was then added after induction of platelet aggregation by ADP at 3 min, and the resultant dissociation of the platelet aggregates was measured by the decrease in optical density of the sample. The addition of increasing amounts of the HB CAN after initiation of platelet aggregation resulted in increasing platelet disaggregation. Wild-type human CAN (red trace) lacks the ability to reverse platelet aggregation, even at a concentration of 400 µg/ml. Reprinted from Cell, Vol. 116, Dai et al., Structure and Protein Design of a Human Platelet Function Inhibitor, pp 649–659, Copyright (2004), with permission from Elsevier.
