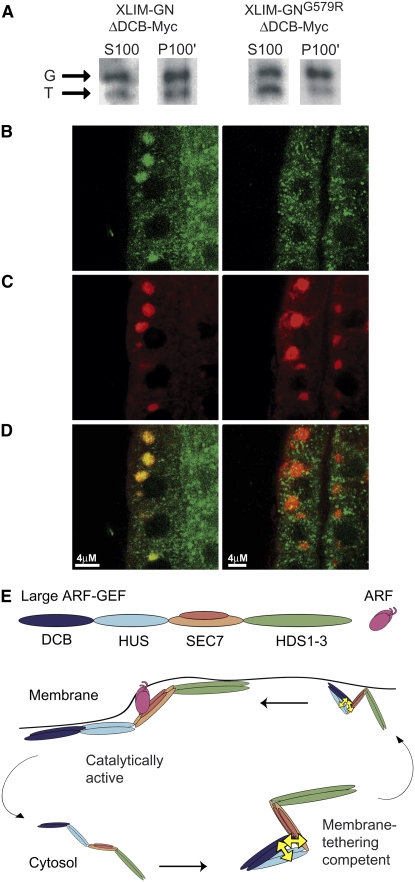
Figure 5.
The N-Terminal Part of the SEC7 Domain Is Required for Membrane Association of GNOM.
(A) to (D) Membrane association of XLIM-GNOMΔDCB-Myc and XLIM-GNOMG579RΔDCB-Myc expressed as transgenes in wild-type seedlings.
(A) Subcellular fractionation of soluble (S100) and membrane (P100') fractions detected with anti-SEC7-antiserum; P100' was loaded in 10-fold excess of S100. Arrows: endogenous full-length GNOM (G); internal control, transgenically produced truncated GNOM (T). Note difference in S100/P100' ratio of GNOMΔDCB and GNOMG579RΔDCB.
(B) to (D) Subcellular localization of XLIM-GNOMΔDCB-Myc (left panels) and XLIM-GNOMG579RΔDCB-Myc (right panels) in BFA-treated seedling roots.
(B) Myc staining of transgenic proteins (green).
(C) ARF1 staining of BFA compartments (red).
(D) Double labeling of Myc and ARF1 (overlay).
(E) Regulation of large ARF-GEF function by heterotypic interaction. Cyclic changes of ARF-GEF conformation from a form competent for membrane tethering (right) to an open catalytically active form that is effectively membrane associated (left), which is followed by dissociation from the membrane and reestablishment of the heterotypically interacting form. Double-headed arrows (yellow) indicate the heterotypic interaction of the DCB domain with the HUS and SEC7 domains. The model depicts the heterotypic interaction as intramolecular and the large ARF-GEF as a constitutive dimer but does not pretend to reflect the actual as yet unknown structural changes of GNOM.