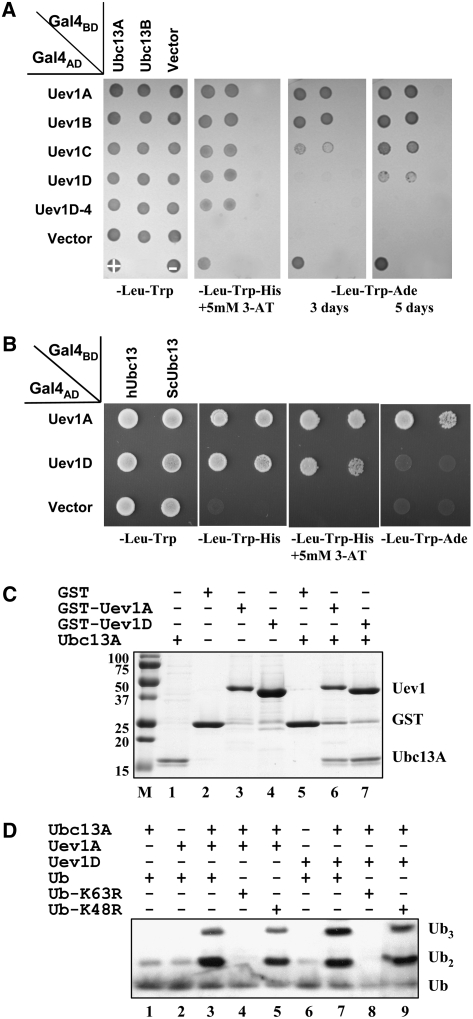
Figure 2.
Biochemical Properties of Uev1.
(A) Physical interaction between Ubc13 and Uev1 in a yeast two-hybrid assay. The PJ69-4A transformants carrying one Gal4AD (from pGAD424) and one Gal4BD (from pGBT9) were replicated onto various plates as indicated and incubated for 3 d or as specified before being photographed. The result is representative of at least five independent transformants from each treatment.
(B) Physical interactions between At Uev1A/D and Ubc13 from yeast or human in a yeast two-hybrid assay. Experimental conditions were the same as in (A).
(C) Protein interactions between Uev1A/D and Ubc13 by an affinity pull-down assay. Purified GST (lane 5), GST-Uev1A (lane 6), or GST-Uev1D (lane 7) was added to GST microspin columns. Following incubation, the columns were spun and washed, and purified Ubc13A was added to the column. After reincubation and washing, the column contents were eluted with reduced glutathione, followed by SDS-PAGE gel analysis. Lanes 1 to 4 contain purified input proteins as indicated at top. Note that spontaneous cleavage occurred in the two GST-Uev1 protein samples (lanes 3 and 4).
(D) Ub conjugation by Ubc13, Uev1A, and Uev1D. An in vitro Ub conjugation assay was performed using purified proteins as indicated. Assay samples were subjected to SDS-PAGE, and a protein gel blot using an anti-Ub antibody was assayed to monitor poly-Ub formation. The low background of spontaneously formed di-Ub in the absence of E2 or Uev (lanes 1, 2, and 6) is commonly observed in these reactions (McKenna et al., 2001).