Abstract
It has recently been demonstrated that developmental exposure to methylmercury (MeHg) is associated with perseveration on operant tasks. An understanding of the behavioral mechanisms underlying this phenomenon may improve human testing of MeHg exposures and could provide insight into clinical syndromes that include perseveration as a component. One possible mechanism is that MeHg-induced enhancement of reinforcer efficacy produces a “reinforcement trap” that inhibits change in novel situations. Rats were exposed gestationally to 0, 0.5 or 5 ppm mercury (Hg) as MeHg via maternal drinking water. They also received a diet during gestation and throughout life that was marginal (0.06 ppm) or rich (0.6 ppm) in selenium (Se), a nutrient believed to protect against MeHg's toxicity. Reinforcer efficacy was evaluated using a progressive ratio schedule of reinforcement during adulthood. Maximum ratio obtained (MRO) was determined using 20 or 60 mg sucrose pellets and with ratio requirements that increased at 5% or 20% per reinforcer. MRO was related to the rate at which the ratio increased, reinforcer magnitude, sex, and exposure regimen; MRO was increased for the 0.6 ppm Se, 5 ppm Hg group. This extends an earlier observation that developmental MeHg exposure enhances reinforcer efficacy, an effect that could be related to reports of perseveration.
Keywords: methylmercury, selenium, progressive ratio, developmental, operant behavior
1. Introduction
In animal models, gestational MeHg exposure alters behavioral sensitivity to changes in reinforcement, effects that extend well into adulthood despite cessation of exposure after birth. This has been reported in monkeys [30] and rats [29] using concurrent schedules of reinforcement to examine the acquisition of choice between two subtly different outcomes. Deficits have also been demonstrated in discrimination reversal [34] and spatial alternation tasks [43]. The pattern of errors seen in both the discrimination reversal [34] and spatial alternation tasks [43] suggests that the rats perseverated on the lever that was previously associated with reinforcement. These data suggest the presence of a MeHg-induced behavioral rigidity that is expressed after reinforcement contingencies are altered.
Further evidence of perseverative responding in MeHg-treated animals comes from studies of responding maintained under fixed-ratio (FR) schedules of reinforcement. Acquisition of lever-pressing under large FR schedules of reinforcement is usually accompanied by a weak and erratic response pattern called “ratio strain,” in which previously high rates of responding disintegrate when the FR requirement is increased, but in animals exposed prenatally to MeHg, responding proceeded more robustly than in controls [31]. In that experiment, subjects responded under a FR1, FR5, FR25, and FR75 (i.e., 1, 5, 25, and 75 responses/reinforcer) for three days each. Control animals exhibited ratio strain, especially during the transition to the FR 75 condition, whereas MeHg-exposed animals showed high response rates under the FR 75 schedule as though the response requirement had not been changed [31].
This general effect was reproduced in the same group of subjects under various progressive ratio (PR) schedules of reinforcement [31] in which the ratio requirement increased by 5, 10 or 20% after each reinforcer. Methylmercury-exposed rats completed more ratios, attained a greater maximum ratio, and responded at higher rates throughout the series of FR schedules than the controls, with statistically significant differences seen during the 5% session. Because PR schedules are used to quantify reinforcer efficacy [38], these results suggest an increase in reinforcer efficacy among MeHg-treated animals. This account is consistent with the hypothesis that animals perseverate on a previously reinforced response alternative because an increased impact of reinforcement would yield persistent responding on a previously rich alternative in concurrent schedule [29,30] and discrimination reversal [34] procedures.
In the study of FR and PR schedules described above [32], subjects were maintained on a diet that was either high or low in the n-3 polyunsaturated fatty acid docosahexaenoic acid (DHA) throughout life, but neither a main effect of DHA nor interactions with MeHg were noted. Another nutrient that may play a role in modulating the developmental neurotoxicity of MeHg is selenium (Se), which has antioxidant properties and binds mercury (Hg). With respect to the latter effect, Se may reduce MeHg's neurotoxicity by sequestering the inorganic form of the metal once it enters the brain. Alternatively, it has been hypothesized that these Hg-Se bonds are responsible for the neurotoxic effects of MeHg because they could reduce the amount of bioavailable Se [32], functionally resulting in a Se deficiency. Increasing the levels of Se in the diet might allow for a molar excess of Se over Hg, thereby decreasing MeHg's developmental neurotoxicity.
The present study was designed to test two hypotheses. The first is that developmental MeHg exposure alters reinforcer efficacy as determined with progressive ratios of reinforcement. Since responding should be positively correlated with reinforcement magnitude [38], the validity of the progressive ratio as a measure of reinforcing efficacy was verified by explicitly varying reinforcement magnitude. We also expanded on previous research [31] by varying the rate at which response requirements increased. The second hypothesis was that Se alters MeHg's developmental neurotoxicity. Subjects were exposed to MeHg during gestation via maternal drinking water that contained 0, 0.5, or 5 ppm of Hg as MeHg. They were also exposed to a diet containing 0.06 or 0.6 ppm of Se, levels that are low and high in Se, respectively, but that result in neither a deficiency nor a toxic excess of Se.
2. Methods
2.1 Subjects
The subjects were 53 Long-Evans rats (F1 generation) (27 males, 26 females) exposed in utero to MeHg via maternal consumption of drinking water containing 0, 0.5, or 5 ppm of Hg as methylmercuric chloride. Both breeders and offspring consumed an AIN-93 purified diet containing approximately 0.06 or 0.6 ppm Se throughout life. This formed a 2 (chronic Se) × 3 (developmental MeHg) factorial design. Each group contained between 8 and 11 subjects (4−5 females per group; see Table 1). Details are provided below.
Table 1.
Group composition by Hg, Se, and Sex
| |
Selenium |
|
|---|---|---|
| Methylmercury (ppm) | Low | High |
| 0 | 5 (F) | 4 (F) |
| 6 (M) | 4 (M) | |
| 0.5 | 4 (F) | 4 (F) |
| 4 (M) | 4 (M) | |
| 5.0 | 5 (F) | 4 (F) |
| 5 (M) | 4 (M) | |
All rats were housed in environmentally controlled colony rooms with a 12:12 light-dark cycle (lights on at 7:00 a.m.). They were monitored daily by research staff and personnel from the Department of Laboratory Animal Health at Auburn University and were inspected by veterinary staff at least twice a week. Sentinel rats exposed to the same air and bedding taken from selected rats used in the study were inspected semiannually for infectious diseases. All procedures were approved by the Auburn University Institutional Animal Care and Use Committee. The colony was housed in an AAALAC-accredited facility that also met PHS guidelines for animal care.
2.2 Selenium Exposure: (Breeders and Offspring diets)
At 18 weeks (125 days) of age, the breeders (F0 generation; Harlan, Indianapolis, IN) of the female rats used in the present experiment were placed on one of two diets, each based on the AIN-93 formula for laboratory rodents but customized for Se concentration (see Figure 1). The “low selenium” diet contained naturally available Se from casein only at a nominal concentration of 0.06 ppm. This is a low but still nutritionally adequate level of Se for rodents [25,36]. The “high selenium” diet was supplemented with sodium selenite to produce a Se level of 0.6 ppm. The higher concentration represents an excess over the standard AIN-93 formulation, which normally contains 0.15 ppm of Se [35,36], but is below that thought to be toxic [1]. Dietary Se was derived from casein and can vary somewhat, so Se content of the diets was analyzed using inductively coupled plasma mass spectrometry (ICP-MS). Actual Se concentrations were between 0.05 and, in one shipment used for adult consumption, 0.1 ppm in the low-Se and 0.6 and 0.9 ppm in the high-Se diets.
Figure 1.

Timeline for breeding and exposure for F0 breeders and F1 offspring. Note that maternal exposure to MeHg ended at 16 days and was reinstated after weaning. Functionally, exposure of the F1 generation to MeHg ended at birth because little MeHg is passed via lactational transfer [28,39].
Between mating and lactation, female breeders were fed a base diet of AIN-93 growth diet containing 7% fat from soybean oil. A maintenance diet of an AIN 93 diet with 4% fat was used at all other times. Both diets were obtained from Research Diets Inc. (New Brunswick, NJ). Dietary Hg was below the detectable level of 50 ppb. Male breeders were maintained on a standard chow diet except when briefly exposed to the females' diet during breeding (see Breeding). All F1 offspring received the same diet as their mothers throughout life.
2.3 Methylmercury Exposure: Breeders only
At approximately 21 weeks (145 days) of age, following three weeks (20 days) on custom Se diets, each Se group of F0 female breeders was further divided into three MeHg exposure groups to create 6 experimental groups of approximately equal body weight. Methylmercury was added to the drinking water of female F0 breeders in concentrations of 0, 0.5, or 5 ppm of Hg as methylmercuric chloride (Alfa Aesar, Ward Hill, MA; hereafter groups are referred to as 0, 0.5, or 5 ppm Hg). These concentrations produce exposures of about 0, 40 and 400 μg/kg/day respectively, based on average daily consumption, with some elevation during gestation due to increased fluid intake [28]. Sodium carbonate (< 5 nanomolar), which can buffer MeHg [39], was added to all three water mixtures. Maternal exposure to MeHg-containing water was discontinued on PND 16 when the F1 pups were capable of reaching the waterspout. Because there is little exposure via breastmilk, MeHg exposure functionally terminated at birth [28,39]. Throughout the remainder of life, all F1 rats received tap water to drink. Male breeders received exposure to tap water only.
2.4 Breeding
Beginning at approximately 23.5 weeks of age (following 2.5 weeks of MeHg exposure and 5.5 weeks of Se exposure) and continuing to 42 weeks of age, male and female Long-Evans rats (F0 generation; Harlan, Indianapolis, IN) were mated. Female breeders were assigned to exposure groups unevenly in anticipation of exposure-related differences in reproductive success. The low Se diet groups contained 16, 19, and 34 breeders in the 0, 0.5, and 5.0 ppm groups respectively. The high Se diet groups contained 12, 16, and 18 breeders respectively. Females were weighed 3 days/week after mating, and if they failed to gain weight, showed sudden weight loss that implied litter loss, or had evidence of litter loss in their home cage then they were rebred after at least two weeks had passed. Two factors contributed to the long breeding process: matings were planned to spread births over a period of several weeks, and both low Se status and high MeHg exposure contributed to difficulties in carrying a litter to term.
Females' home cages were used as breeding cages and contained the female's diet and tap water so that males were never exposed to MeHg. Each male was paired with a single female during every other dark cycle. Most males were paired with a second female during alternating dark cycles. A male was paired with the same female(s) throughout breeding. When a male was mated with two females, the females were always members of different exposure groups. Breeding of females continued until a sperm plug or systematic increases in daily body weight was observed, suggesting gravidity. Births before 5:00 pm were assigned to PND 0 for that day. All births after 5:00 pm were assigned to PND 0 for the subsequent day. Large litters were culled and small litters combined to produce 8 F1 pups of the same age and exposure group, including at least three females when possible, but only one female or male per litter was randomly selected for inclusion in the present study. None of the cross-fostered pups were selected for inclusion in behavioral testing.
After weaning on postnatal day (PND) 21, offspring (F1 generation) were injected subcutaneously with an electronic identification chip (Biomedic Data Systems, Seaford, DE). Subjects were housed two per cage in 22 × 20 × 45cm plexiglass shoebox cages with wire tops and free access to water, but were kept separate by a transparent divider placed diagonally in the cage. Rats that shared a home cage were of the same sex and received the same exposure regimen. After PND 90, rats were weighed daily and food was rationed to approximately 10 gm/day to maintain the body weight at 250 grams for females and 300 gm for males. To prevent excessive tooth growth, a cleaned, nylon chew “bone” was freely available in the home cage. Subjects were 8 ± 1 months of age at the beginning of the present experiment.
2.5 Testing Apparatus
Experiments were conducted in 16 commercially available operant chambers (Med-Associates, Model ENV-008) containing one rear-mounted lever and two front, retractable levers calibrated so that 0.20 Newton (20 gm mass) registered a response, a pellet dispenser situated between the two front levers that delivered 20 mg sucrose pellets (Research Diets, Inc., New Brunswick, NJ), Sonalert tones™ (2900 and 4500 Hz, nominally; adjusted to an amplitude of 70 dbC), a house light (28 V 100 ma), and a light emitting diode (LED) above each lever. Drinking water was freely available through a custom mounted bottle with a spout located to the left of the rear lever. Each chamber was surrounded by a sound-attenuating cabinet with a built-in ventilating fan that circulated air into the experimental environment and provided masking noise. Programs for experimental procedures and data collection were written using MED-PC IV (Med-Associates, Georgia, VT). Session events were recorded with 0.01” resolution.
2.6 Behavioral Methods
At the beginning of the study and throughout experimental testing, body weights did not differ among any of the exposure groups. Each of four squads of subjects, consisting of 16 animals each, was tested sequentially beginning at 9 a.m. with each squad being tested at approximately the same time every day Monday through Friday. Assignment of subjects to squads and chambers was distributed across exposure groups. Fans, lights, tones, levers, and pellet dispensers were tested before and after sessions for each squad of rats to ensure that equipment was functioning properly. Electronic identification chips were scanned prior to each session to insure rats were placed in the appropriate chamber and home cage. Neither squad nor chamber had systematic effects on behavior, as determined by one-way ANOVAs conducted periodically during the experiment.
Subjects were trained to lever-press using an autoshaping procedure [9,29] at 6±1 months of age. Initially, rats received magazine training in which 12 sucrose pellets were delivered according to a fixed-time (FT) 60-sec schedule and paired with a 0.5 sec, 4500 Hz tone, which signaled the delivery of a reinforcer. After the delivery of 12 noncontingent sucrose pellets, the autoshaping procedure was in effect. At the onset of the autoshaping procedure, the right lever extended (the left lever was always retracted), the right stimulus light was lit, and a 0.5 sec, 2900 Hz tone was sounded with the extension of the lever. During this time, a fixed-ratio (FR) 1 schedule of reinforcement was in effect in which a single lever-press resulted in the delivery of a sucrose pellet and a 0.5 sec, 4500 Hz tone was sounded. There was no change in the stimulus light. After 30 seconds elapsed, the lever retracted, the stimulus light turned off, a 0.5 sec, 4500 Hz tone sounded and one noncontingent sucrose pellet was delivered. Subsequent trials lasted 30 seconds, and each intertrial interval (ITI) lasted 4.5 minutes, during which time the lever was retracted and the stimulus light was off. The autoshaping component ended after 10 responses were made, and training was considered complete once 100 additional responses were made on the lever.
A progressive ratio (PR) requirement was then imposed. Subjects were tested under three different PR conditions. During phase 1, the response requirement for the first reinforcement on day 1 was 1 response, and the requirement was increased by 5% or one response, whichever was greater, after each subsequent reinforcer delivery. Reinforcement consisted of one, 20 mg sucrose pellet. For the remainder of phase 1, the response requirement at the beginning of each session was set to half of the maximum ratio obtained (MRO) from the previous day. Each session lasted two hours regardless of responding. Phases 2 and 3 were identical to phase 1 with the following exceptions: (1) reinforcers in phase 2 consisted of three 20 mg sucrose pellets (yielding a 60 mg reinforcer) and (2) the response requirement was increased by 20% for each subsequent reinforcement in phase 3, but the reinforcer was a single sucrose pellet. Phases 1, 2, and 3 lasted 7, 8, and 5 sessions, respectively (see Table 2). Due to time constraints, phase length was determined by the presence of stable responding, which was defined as a change in mean MRO value of less than ten for three consecutive days by control subjects. The dependent variable for each phase was the MRO at the end of each session.
Table 2.
Duration, response requirement, and reinforcer magnitude for each phase
| Response Requirement | Reinforcer Magnitude | ||
|---|---|---|---|
| Phase | Sessions | Increment | (mg of sucrose) |
| Phase 1 | 1−7 | 5% | 20 mg |
| Phase 2 | 8−15 | 5% | 60 mg |
| Phase 3 | 16−20 | 20% | 20 mg |
2.7 Data and Statistical Analyses
All statistical analyses were performed using Systat® 11 (Systat Software, Inc. Point Richmond, CA). The Type 1 error rate (α) was set to 0.05 for all tests. A repeated-measures analysis of variance (RMANOVA) was performed for each phase of the experiment with MeHg (0, 0.5, 5 ppm), Se (low, high), and sex (male, female) serving as the three between-subjects factors with 4−6 rats per cell (see Table 1). Session served as the repeated, within-subject factor. Each omnibus repeated-measures ANOVA permitted the detection of seven overall, between-subjects effects (MeHg, Se, sex, MeHg* Se, MeHg*sex, Se*sex, and MeHg*Se*sex) and eight within-subject effects (session, session*MeHg, session*Se, session*sex, session*MeHg*Se, session*MeHg*sex, session*Se*sex, and session*MeHg*Se*sex). To assess the effects of phase, a repeated-measures ANOVA was conducted using the last session of each phase as the dependent variable. The Huynh-Feldt correction was used when necessary to adjust the degrees of freedom for the tests of within-subject effects.
Significant ANOVAs were followed by pairwise, Tukey post hoc comparisons. To examine Se by MeHg interactions, comparisons of each MeHg group were made across Se diets. In addition, each MeHg group was compared with its appropriate dietary control for all phase-diet blocks. F- ratios, degrees of freedom, and p-values were reported for all RMANOVAs and two-way ANOVAs. For post-hoc contrasts and comparisons, p-values were reported only in figures.
3. Results
3.1 Reproductive success
Animals that failed to carry a litter to term were rebred until an adequate sample size was obtained. In the high Se group there were 10/12 (litters/number of breeders), 13/16 and 11/18 litters for the 0, 0.5, and 5 ppm exposure groups, respectively. In the low Se group there were 12/16, 12/19, and 18/34 litters. Log-linear analyses revealed no interaction between MeHg and Se, but there was a main effect of MeHg (P=0.02) and of Se (P=0.03) on reproductive success.
3.2. Phase 1: PR 5%, 1 Reinforcer
MRO increased as a function of session [F(6,246) = 29.12, P<0.001] (see Figure 2). There was a between-subjects effect of MeHg [F(2,41)=3.3, P=0.047] such that the 5 ppm Hg group responded more than both the controls and 0.5 ppm Hg group. This is particularly evident during the later sessions although post hoc comparisons were not conducted since there was no interaction with session. No other statistically significant between- or within-subjects effects or interactions were observed (Ps>0.1).
Figure 2.

Phase 1: 5% PR, 20 mg sucrose reinforcer. Maximum ratio obtained (MRO) for 0.0 ppm Hg group (filled circles), 0.5 ppm Hg group (open squares), and 5 ppm Hg group (open triangles), combined across selenium exposure, for each session. Error bars represent ± 1 SEM. (p<0.05 between-subjects effect of Hg (##)).
3.3. Phase 2: PR 5%, 3 Reinforcers
There was a within-subject effect of session [F(7,287)=30.18, P<0.001] on MRO (see Figure 3). Responding increased from sessions 8−10 for all groups, at which point responding plateaued for the controls and 0.5 ppm Hg group. For animals exposed to 5 ppm Hg, responding from sessions 13 − 15 was influenced by dietary Se. MRO values for the high Se, 5 ppm Hg group continued to increase across sessions, resulting in significantly greater responding compared to both their dietary controls and the low Se, 5 ppm Hg group, whose MRO values decreased in later sessions. This was manifested statistically as an interaction among sessions, Se, and MeHg [F(14,287)=2.23, P=0.024]. There were no between-subjects effects of sex, Se, or MeHg and no interaction among them (Ps>0.1).
Figure 3.
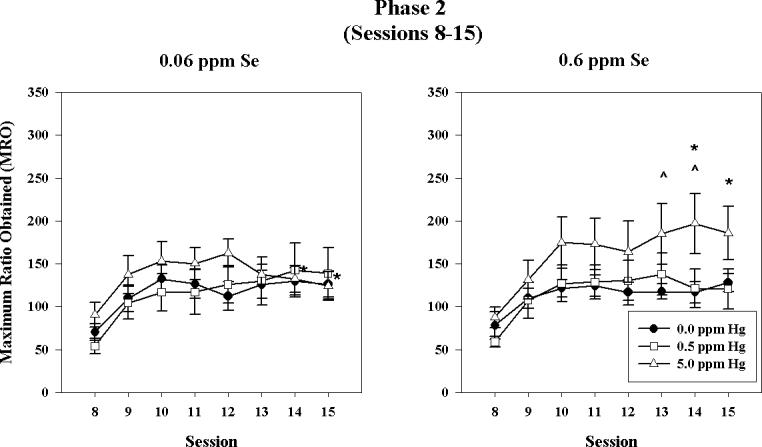
Phase 2: 5% PR, 60 mg sucrose reinforcer. Maximum ratio obtained (MRO) for 0.0 ppm Hg group (filled circles), 0.5 ppm Hg group (open squares), and 5 ppm Hg group (open triangles) for the low Se (left) and high Se (right) groups across sessions. Error bars represent ± 1 SEM. (p < 0.05 post hoc comparison; (*) 0.06 ppm Se, 5.0 ppm Hg vs. 0.6 ppm Se, 5.0 ppm Hg; (^) 0.6 ppm Se, 0.0 ppm Hg vs. 0.6 ppm Se, 5.0 ppm Hg).
3.4. Phase 3: PR 20%, 1 Reinforcer
There was a main effect of session [F(4,164)=8.43, P<0.001] (see Figure 4) and an interaction between session and sex [F(4,164)=2.9, P=0.038]. MRO was slightly higher for males than females at the beginning of phase 3, but values eventually converged in later sessions due to a decrease in males' responding (not shown). There was no interaction between sex and either MeHg or Se treatment (Ps>0.1).
Figure 4.

Phase 3: 20% PR, 60 mg sucrose. Maximum ratio obtained (MRO) for 0.0 ppm Hg group (filled circles), 0.5 ppm Hg group (open squares), and 5 ppm Hg group (open triangles) for the low Se (left) and high Se (right) groups across sessions. Error bars represent ± 1 SEM. (p < 0.05 post hoc comparison; (*) 0.06 ppm Se, 5.0 ppm Hg vs. 0.6 ppm Se, 5.0 ppm Hg; (^) 0.6 ppm Se, 0.0 ppm Hg vs. 0.6 ppm Se, 5.0 ppm Hg).
Subjects in the low Se, 0.5 ppm Hg group showed a decrease in MRO values across sessions. In contrast, subjects in the high Se, 5 ppm Hg group showed an increase in MRO values as a function of session, revealing a significant interaction among session, MeHg, and Se [F(8,164)=2.805, P=0.014]. In addition, for animals maintained on the low Se diet, MRO values were higher for subjects exposed to 0.5 ppm Hg than for subjects exposed to 5 ppm Hg, while the reverse was true for the high Se group, creating an interaction between MeHg and Se [F(2,41)=3.37, P=0.04]. One animal in the low Se, 0.5 Hg ppm exposure group was identified statistically as an outlier. Its influence can be seen in the elevated mean and larger error bars for that exposure group. The statistical analysis was re-conducted with data from this subject omitted, but the results did not change substantively. Graphically, removal of this outlier is manifested as MROs that more closely approximate those observed in the other two groups maintained on a low Se diet.
3.5. Effect of Phase
There was a main effect of phase [F(2,82)=40.82, P<0.001] such that responding increased as a function of phase, with the exception of the low Se, 5 ppm Hg group, for which MRO values were similar across phases (see Figure 5). The high Se, 5 ppm Hg group displayed a much steeper increase in MRO values as compared to the other groups. This resulted in an interaction among phase, MeHg, and Se [F(4,82)=6.80, P<0.001]. There were no between-subjects effects of MeHg, Se, or sex and no interaction among them (Ps>0.1).
Figure 5.

Maximum ratio obtained (MRO) for the last session of each phase as a function of MeHg exposure and selenium. Error bars represent ± 1 SEM. (For simplicity, post hoc comparisons were conducted within, not across, each phase. p < 0.05 post hoc comparison; (*) 0.06 ppm Se, 5.0 ppm Hg vs. 0.6 ppm Se, 5.0 ppm Hg; (^) 5.0 ppm Hg vs. its dietary control).
4. Discussion
The present study was designed to examine the behavioral consequences of gestational exposure to MeHg and a lifelong diet that was either marginal or rich in Se. Selenium and MeHg were manipulated in a 2 (Se) × 3 (MeHg) × 2 (sex) factorial design, so main effects of the elements, as well as their potential interaction, could be detected. Animals exposed to MeHg during gestation showed enhanced responding on a PR schedule of reinforcement in Phase 1, during which the ratio requirement increased by 5% after each reinforcer and 20 mg pellets were used. In Phase 2, when the reinforcer magnitude was relatively large, and in Phase 3, when the ratio value increased rapidly, an interaction with Se was detected.
The present investigation extends a previous one in which gestational exposure to MeHg facilitated responding on ratio schedules of reinforcement as the response requirement increased rapidly [31]. In that study, dietary levels of n-3 polyunsaturated fatty acids were varied between groups, and the manner in which ratio schedule increased differed from the present one. The results for phase 1 (PR 5%) are in accordance with what was observed in the previous study; MeHg-exposed animals, regardless of dietary exposure, responded more than controls. However, the results for phase 3 (PR 20%) differ, probably due to procedural differences [31]. For example, rats in the previous study experienced each condition for only one day, while conditions in the current study were presented for several days. The importance of this difference is demonstrated by the fact that distinctions among the exposure groups increased across sessions in the present study. In addition, the present animals were naïve at the beginning of the study, whereas subjects in the Paletz study had experience responding under FR and differential reinforcement of low-rate behavior schedules, the latter of which immediately preceded PR testing. Despite these differences, response patterns and overall rates were similar across studies.
In the present experiment, the utility of the PR as a measure of reinforcer efficacy [8,16,18,38] was confirmed in phase 2 in which the reinforcer magnitude increased from 20 to 60 mg while the PR schedule changed at the same rate (5%/reinforcer) as in phase 1. The 60 mg sucrose reinforcer supported higher MROs than did a 20 mg reinforcer. The observation that some MeHg-exposed animals had larger MROs than unexposed animals suggests that this increased reinforcer magnitude had an even greater effect in those animals. The results of phase 3 provide even stronger evidence of a more potent effect of reinforcement on the response that produced it in MeHg exposed rats, particularly those exposed to high Se and in later sessions. In this last phase, the reinforcer magnitude decreased even as the PR value increased more rapidly (20%) than in phase 2. Of the three phases, this last phase most closely resembles the rapid acquisition of FR responding in Paletz et al. [31]. The observation that responding among MeHg-treated animals remained robust while responding decreased in control groups may appear counterintuitive, but similar results have been observed following developmental exposure to cadmium [27] and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) [17].
The preceding effects may be described as a “reinforcement trap” in which enhanced reinforcer efficacy produces such strong responding that behavior becomes rigid and resistant to change after a decrease in reinforcer density. This hypothesis is supported by observations that MeHg-exposed animals show retarded transitions under concurrent schedules [29,30], have increased error rates in spatial discrimination reversals [34], demonstrate robust responding under both FR and PR schedules when compared with controls [31], and may have difficulty acquiring responding under DRL schedules of reinforcement following a history of high-rate responding [30]. All of these are effects that would be expected if reinforcers had a more powerful effect on the behavior of MeHg-exposed rats as compared to that of control animals.
These behavioral changes may be associated with MeHg-induced insult to the frontal cortex or to meso-cortical dopamine pathways [26]. The activity of cortical dopamine neurons discriminates between two reinforcement alternatives and reflects an animal's preference in a choice setting, suggesting that these neurons process the relative value of reinforcement [40,41]. Developmental MeHg exposure alters the synaptosomal uptake of dopamine [3], dopamine transmitter levels and turnover [3,4], and reduces monoamine oxidase (MAO) activity [7], alterations that may increase synaptic dopamine levels and thereby increase reinforcer efficacy [15,44]. These synaptic effects are reflected in elevated sensitivity of the behaving animal to acute doses of amphetamine in young rats [12,18]. Moreover, such sensitivity also appears in fully grown animals and is specific to amphetamine (which interacts with dopaminergic systems), but not to other behaviorally active drugs that act elsewhere [33].
Exposure to MeHg in utero also produces morphological changes in, and deranged lamination of, cortical neurons [2,6]. Cortical lesions have been associated with alterations in PR performance and with impulsive and perseverative behaviors in animals [13,19,20,22,23] and humans [14,33]. Excitotoxic lesions to the orbital prefrontal cortex and lesions to the frontal lobes produce perseveration on discrimination reversal tasks in rats and marmosets [11,37], respectively. The present study, and these other reports, support a hypothesis that the perseverative behavior observed in MeHg-exposed animals is the result of damage to the cortex and/or alterations in the dopaminergic neurotransmitter system, which alters the sensitivity of behavior to reinforcing consequences.
4.1. MeHg and Selenium Interactions
Selenium is thought to play an important role in CNS functioning, but its precise mechanisms are dimly understood. Evidence of Se's importance lies in the aggressive defense of CNS concentrations of Se, even in the face of severe dietary depletion and at the expense of other organs [5,10]. With respect to MeHg neurotoxicity, Se could exert its effects by binding Hg to form insoluble complexes that diminish the MeHg's biological effects. These bonds could, however, also render Se unavailable for biologically important selenoproteins, such as glutathione peroxidase.
Selenium delays the onset of some neurological signs of chronic, adult-onset MeHg exposure [21,24], but its role in MeHg's developmental neurotoxicity is not well characterized. In a study using Se levels similar to those in the present study, but somewhat higher MeHg levels, Se deficiency and gestational MeHg exposure were linked to delays in neurobehavioral endpoints such as thermal preference, locomotor activity, and gait in young rats [42]. In that study, the effects of MeHg and Se-deficiency combined additively, implying that each had a separate effect on the endpoints studied. No statistical interaction between MeHg and Se was reported.
In phase 1 of the current study, we observed higher MRO values in those rats exposed to 5.0 ppm MeHg, regardless of dietary Se exposure, in acquisition curves (see Figure 2). In phases 2 and 3, however, increased MRO values were seen only in the high Se, 5.0 ppm MeHg group. The summary measures (see Figure 5), taken from the last sessions under each PR condition, also displayed an interaction. Because these increases were correlated with prolonged experience under a PR schedule, it might be suggested that changes in MRO were a result of experience rather than increased sensitivity to reinforcement or decreased sensitivity to increasing response requirements. Two observations make this questionable. First, changes in reinforcer magnitude and schedule parameters were made only after responding had stabilized. Therefore, increases in MRO were observed only after the independent variables changed. Second, where increases between phases were observed in other groups, they were not of the magnitude seen in the high Se, 5.0 ppm MeHg group. In fact, responding in the low Se, 5.0 ppm MeHg group remained stable across all three phases of the experiment. If increases in MRO were a simple function of experience, we would expect to see similar changes among all exposure groups. In addition, while we observed increases in MRO from phase 2 to 3 among high Se, 5.0 ppm MeHg animals, we failed to see similar changes in the control group.
The reason for the complex interaction between gestational MeHg and dietary Se is not clear, but it is noteworthy that an interaction between developmental MeHg exposure and lifetime Se exposure exists. In contrast, there was no interaction between MeHg exposure and a diet that was rich in n-3 polyunsaturated fatty acids in a similarly designed experiment [31]. Further, there was no interaction between MeHg and Se on spatial discrimination reversals among littermates of the rats described in the present study, but there was a main effect of Se on failures to complete a trial [34]. Thus, it appears that dietary Se is behaviorally active and may have subtle interactions with MeHg exposure, but the precise nature of these interactions are difficult to ascertain at present.
4.2. Concluding Remarks
The present results suggest that MeHg-exposure produces altered sensitivity to reinforcers and may provide a behavioral mechanism by which developmental MeHg exposure produces behavioral rigidity and perseveration. By enhancing reinforcer efficacy, it may trap behavior and make it less likely to change when required to do so.
ACKNOWLEDGEMENT
Supported by NIH ES 10865. The research was supported by a grant from the National Institutes of Health. After deciding to support the project from which this work came, the sponsor had no involvement in the collection, analysis and interpretation of the data.
This work was supported by ES10865 from NIEHS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT: My coauthors and I do not have any interests that might be interpreted as influencing the research.
References
- 1.Abdo KM. NTP technical report on toxicity studies of sodium selenate and sodium selenite, U.S. Department of Health and Human Services. NIH; Research Triangle Park, NC: 1994. [Google Scholar]
- 2.Barone S, Jr., Haykal-Coates N, Parran DK, Tilson HA. Gestational exposure to methylmercury alters the developmental pattern of trk-like immunoreactivity in the rat brain and results in cortical dysmorphology. Dev Brain Res. 1998;109:13–31. doi: 10.1016/s0165-3806(98)00038-8. [DOI] [PubMed] [Google Scholar]
- 3.Bartolome J, Trepanier P, Chait EA, Seidler FJ, Deskin R, Slotkin TA. Neonatal methylmercury poisoning in the rat: effects on development of central catecholamine neurotransmitter systems. Toxicol Appl Pharm. 1982;65:92–9. doi: 10.1016/0041-008x(82)90366-0. [DOI] [PubMed] [Google Scholar]
- 4.Bartolome J, Whitmore WL, Seidler FJ, Slotkin T. Exposure to methlmercury in utero: effects on biochemical development of catecholamine neurotransmitter systems. Life Sci. 1984;35:657–670. doi: 10.1016/0024-3205(84)90261-3. [DOI] [PubMed] [Google Scholar]
- 5.Behne D, Pfeifer H, Rothlein D, Kyriakopoulos A. Cellular and subcellular distribution of selenium and selenium-containing proteins in the rat. In: Roussel AM, Favier AE, Anderson RA, editors. Trace Elements in Man and Animals 10. Kluwer Academic/Plenum Publishers; New York: 2000. pp. 29–34. [Google Scholar]
- 6.Berlin M, Grant CA, Hellberg J, Hellstrom J, Schultz A. Neurotoxicity of methylmercury in squirrel monkeys. Cerebral cortical pathology, interference with scotopic vision, and changes in operant behavior. Arch Environ Health. 1975;30:340–8. doi: 10.1080/00039896.1975.10666717. [DOI] [PubMed] [Google Scholar]
- 7.Beyrouty P, Stamler CJ, Liu JN, Loua KM, Kubow S, Chan HM. Effects of prenatal methylmercury exposure on brain monoamine oxidase activity and neurobehaviour of rats. Neurotoxicol Teratol. 2006;28:251–259. doi: 10.1016/j.ntt.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 8.Bickel WK, DeGrandpre JR, Hughes JR, Higgins ST. Behavioral economics of drug self-administration: II. A unit-price analysis of cigarette smoking. J Exp Anal Behav. 1991;55:145–54. doi: 10.1901/jeab.1991.55-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bushnell PJ. Behavioral effects of acute p-xylene inhalation in rats: autoshaping, motor activity, and reversal learning. Neurotoxicol Teratol. 1989;10:569–577. doi: 10.1016/0892-0362(88)90094-3. [DOI] [PubMed] [Google Scholar]
- 10.Chen J, Berry MJ. Selenium and selenoproteins in the brain and brain diseases. J Neurochem. 2003;86:1–12. doi: 10.1046/j.1471-4159.2003.01854.x. [DOI] [PubMed] [Google Scholar]
- 11.Chudasama Y, Robbins TW. Dissociable contributions of the orbitofrontal and infralimbic cortex to pavlovian autoshaping and discrimination reversal learning: further evidence for the functional heterogeneity of the rodent frontal cortex. J Neurosci. 2003;23:8771–80. doi: 10.1523/JNEUROSCI.23-25-08771.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eccles CU, Annau Z. Prenatal methyl mercury exposure: II. Alterations in learning and psychotropic drug sensitivity in adult offspring. Neurobehav Toxicol Teratol. 1982;4:377–82. [PubMed] [Google Scholar]
- 13.Evenden J. The pharmacology of impulsive behavior in rats V: the effects of drugs on responding under a discrimination task using unreliable visual stimuli. Psychopharmacology. 1999;143:111–122. doi: 10.1007/s002130050926. [DOI] [PubMed] [Google Scholar]
- 14.Head D, Raz N, Gunning-Dixon F, Williamson A, Acker JD. Age-related differences in the course of cognitive skill acquisition: the role of regional cortical shrinkage and cognitive resources. Psychol Aging. 2002:72–84. doi: 10.1037//0882-7974.17.1.72. [DOI] [PubMed] [Google Scholar]
- 15.Heyman GE, Kinzie DL, Seldon LS. Chlorpromazine and pimozide alter reinforcement efficacy and motor performance. Psychopharmacology. 1986;88:346–353. doi: 10.1007/BF00180837. [DOI] [PubMed] [Google Scholar]
- 16.Hodos W, Kalman G. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- 17.Hojo R, Stern S, Zareba G, Markowski VP, Cox C, Kost JT, Weiss B. Sexually dimorphic behavioral responses to prenatal dioxin exposure. Environ Health Persp. 2002;110:247–54. doi: 10.1289/ehp.02110247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hughes JA, Sparber SB. d-Amphetamine unmasks postnatal consequences of exposure to methylmercury in utero: methods for studying behavioral teratogenesis. Pharmacol Biochem Be. 1978;8:365–75. doi: 10.1016/0091-3057(78)90072-2. [DOI] [PubMed] [Google Scholar]
- 19.Kheramin S, Body S, Herrera FM, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. The effect of orbital prefrontal cortex lesions on performance on a progressive ratio schedule: implications for models of inter-temporal choice. Behav Brain Res. 2005;156:145–52. doi: 10.1016/j.bbr.2004.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Kheramin S, Body S, Mobini S, Ho MY, Velazquez-Martinez DN, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of quinolinic acid-induced lesions of the orbital prefrontal cortex on inter-temporal choice: a quantitative analysis. Psychopharmacology. 2002;165:9–17. doi: 10.1007/s00213-002-1228-6. [DOI] [PubMed] [Google Scholar]
- 21.Magos L. Overview on the protection given by selenium against mercurials. In: Suzuki T, Nobumasa I, Clarkson TW, editors. Advances in Mercury Toxicology. Plenum; New York: 1991. pp. 289–298. [Google Scholar]
- 22.Mobini S, Body S, Ho MY, Bradshaw CM, Szabadi E, Deakin JF, Anderson IM. Effects of lesions of the orbitofrontal cortex on sensitivity to delayed and probabilistic reinforcement. Psychopharmacology. 2002;160:290–8. doi: 10.1007/s00213-001-0983-0. [DOI] [PubMed] [Google Scholar]
- 23.Mobini S, Chiang TJ, Ho MY, Bradshaw CM, Szabadi E. Comparison of the effects of clozapine, haloperidol, chlorpromazine and d-amphetamine on performance on a time-constrained progressive ratio schedule and on locomotor behaviour in the rat. Psychopharmacology. 2000;152:47–54. doi: 10.1007/s002130000486. [DOI] [PubMed] [Google Scholar]
- 24.Moller-Madsen B, Danscher G. Localization of mercury in CNS of the rat. IV. The effect of selenium on orally administered organic and inorganic mercury. Toxicol Appl Pharm. 1991;108:457–73. doi: 10.1016/0041-008x(91)90092-s. [DOI] [PubMed] [Google Scholar]
- 25.National Research Council . Nutrient Requirements of Laboratory Animals. Editoin Edition National Academy Press; Washington, D.C.: 1995. [Google Scholar]
- 26.Newland MC, Donlin WD, Paletz EM, Banna KM. Developmental behavioral toxicity of methylmercury: Consequences, conditioning, and cortex. In: Levin ED, Buccafusco JJ, editors. Animal Models of Cognitive Impairment. CRC Press; 2006. pp. 101–146. [PubMed] [Google Scholar]
- 27.Newland MC, Ng WW, Baggs RB, Gentry GD, Weiss B, Millar RK. Operant behavior in transition reflects neonatal exposure to cadmium. Teratology. 1986;34:231–241. doi: 10.1002/tera.1420340302. [DOI] [PubMed] [Google Scholar]
- 28.Newland MC, Reile PA. Blood and brain mercury levels after chronic gestational exposure to methylmercury in rats. Toxicol Sci. 1999;50:106–16. doi: 10.1093/toxsci/50.1.106. [DOI] [PubMed] [Google Scholar]
- 29.Newland MC, Reile PA, Langston JL. Gestational exposure to methylmercury retards choice in transition in aging rats. Neurotoxicol Teratol. 2004;26:179–94. doi: 10.1016/j.ntt.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 30.Newland MC, Yezhou S, Logdberg B, Berlin M. Prolonged behavioral effects of in utero exposure to lead or methyl mercury: reduced sensitivity to changes in reinforcement contingencies during behavioral transitions and in steady state. Toxicol Appl Pharm. 1994;126:6–15. doi: 10.1006/taap.1994.1084. [DOI] [PubMed] [Google Scholar]
- 31.Paletz EM, Craig-Schmidt MC, Newland MC. Gestational exposure to methylmercury and n-3 fatty acids: Effects on high- and low-rate operant behavior in adulthood. Neurotoxicol Teratol. 2006;28:59–73. doi: 10.1016/j.ntt.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 32.Raymond LJ, Ralston NVC. Mercury:selenium interactions and health implications. Seychelles Medical and Dental Journal (SMDJ) 2004;7:72–77. doi: 10.1016/j.neuro.2020.09.020. [DOI] [PubMed] [Google Scholar]
- 33.Raz N, Gunning-Dixon FM, Head D, Dupuis JH, Acker JD. Neuroanatomical correlates of cognitive aging: evidence from structural magnetic resonance imaging. Neuropsychology. 1998:95–114. doi: 10.1037//0894-4105.12.1.95. [DOI] [PubMed] [Google Scholar]
- 34.Reed MN, Paletz EM, Newland MC. Gestational exposure to methylmercury and selenium: effects on spatial discrimination reversal in adulthood. NeuroToxicology. 2006;27:721–732. doi: 10.1016/j.neuro.2006.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127:838S–841S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 36.Reeves PG, Nielsen FH, Fahey GC., Jr. AIN-93 purified diets for laboratory rodents: final report of the American Institute of Nutrition ad hoc writing committee on the reformulation of the AIN-76A rodent diet. J Nutr. 1993;123:1939–51. doi: 10.1093/jn/123.11.1939. [DOI] [PubMed] [Google Scholar]
- 37.Ridley RM, Clark BA, Durnford LJ, Baker HF. Stimulus-bound perseveration after frontal ablations in marmosets. Neuroscience. 1993:595–604. doi: 10.1016/0306-4522(93)90409-9. [DOI] [PubMed] [Google Scholar]
- 38.Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology. 1998;139 doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- 39.Stern S, Cox C, Cernichiari E, Balys M, Weiss B. Perinatal and lifetime exposure to methylmercury in the mouse: blood and brain concentrations of mercury to 26 months of age. Neurotoxicology. 2001;22:467–77. doi: 10.1016/s0161-813x(01)00047-x. [DOI] [PubMed] [Google Scholar]
- 40.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex.[comment] Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 41.Tremblay L, Schultz W. Relative reward preference in primate orbitofrontal cortex.[see comment] Nature. 1999;398:704–8. doi: 10.1038/19525. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe C, Yin K, Kasanuma Y, Satoh H. In utero exposure to methylmercury and Se deficiency converge on the neurobehavioral outcome in mice. Neurotoxicol Teratol. 1999;21:83–8. doi: 10.1016/s0892-0362(98)00036-1. [DOI] [PubMed] [Google Scholar]
- 43.Widholm JJ, Villareal S, Seegal RF, Schantz SL. Spatial alternation deficits following developmental exposure to Aroclor 1254 and/or methylmercury in rats. Toxicol Sci. 2004;82:577–89. doi: 10.1093/toxsci/kfh290. [DOI] [PubMed] [Google Scholar]
- 44.Wise RA. Drug-activation of brain reward pathways. Drug Alcohol Depen. 1998;51:13–22. doi: 10.1016/s0376-8716(98)00063-5. [DOI] [PubMed] [Google Scholar]


