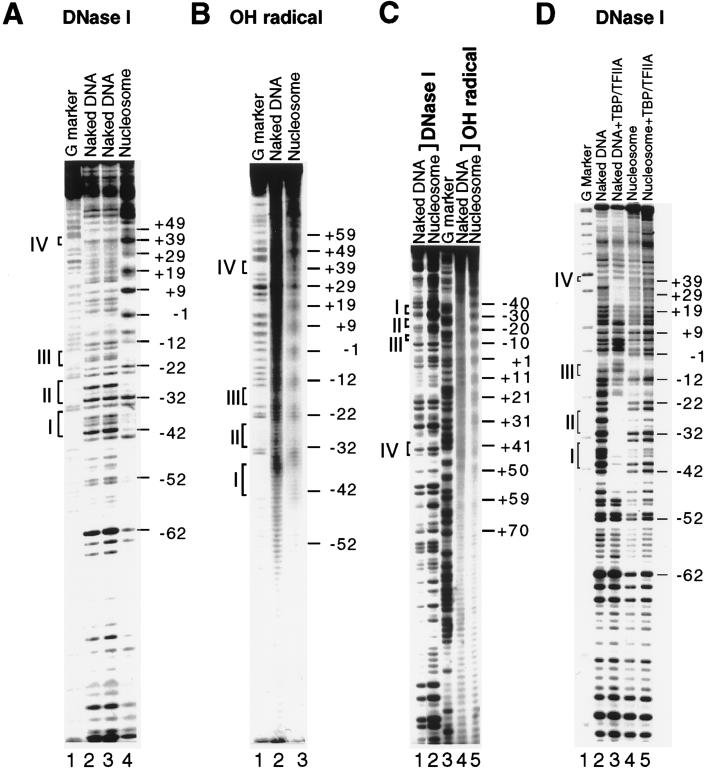
Figure 4.
Nucleosome reconstituted in vitro on a proximal fragment of the phas promoter and its effect on TBP binding. (A) DNase I footprinting of the bottom strand. For DNase I footprinting, the AflIII-XbaI fragment (−112 to +141) from the phas promoter in pBluescript II was reconstituted into nucleosomes and then treated with DNase I (1.5 units). After digestion, the nucleosome-bound fraction was separated by electrophoresis through a 0.7% agarose gel. The relevant band was electroeluted and run on a 6% denaturing acrylamide gel. (B) Hydroxyl radical footprinting of the bottom strand. Nucleosome reconstitution was as in A. For hydroxyl radical footprinting, cleavage was carried out by the generation of radicals through the Fenton reaction. Subsequent steps were as for DNase I footprinting. (C) DNase I and hydroxyl radical footprinting of the top strand of the phas proximal promoter. Footprinting was carried out as in A and B. (D) Incorporation of the phas promoter into a nucleosome prevents the binding of TBP. Equimolar amounts (400 nm) of TBP and TFIIA were added either to naked DNA (lane 3), or to reconstituted nucleosomes (lane 5), and binding was assessed by DNase I footprinting. For A–D, base positions are shown relative to the transcription start site. The phas TATA boxes, shown as brackets I to IV, are positioned at −41 to −35 (TATAATA), −32 to −25 (TATAAATA), −20 to −15 (TAATAT), and +37 to +43 (TATAATA).