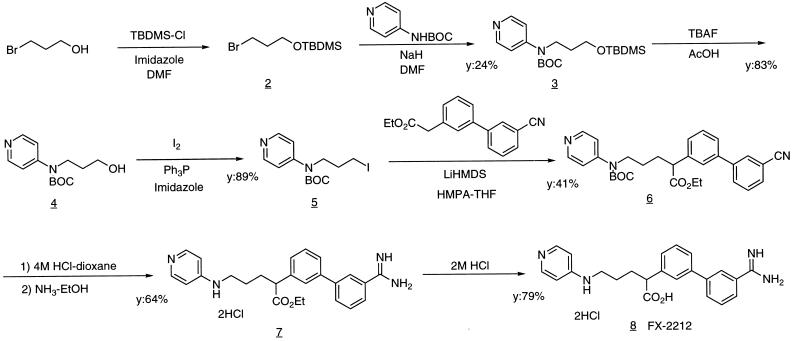
Figure 2.
The scheme of the synthesis of FX-2212. N-tert-Butoxycarbonyl-N-3-(tert-butyldimethylsilyloxy)propyl-4-pyridylamine 3: 2 was prepared from 3-bromo-1-propanol and tert-butyldimethylsilyl chloride. To a solution of 4-tert-butoxycarbonylaminopyridine (400 mg, 2.1 mmol) in N,N′-dimethylformamide (DMF) (4 ml) was added 60% NaH (83 mg, 2.1 mmol) and 2 (1.0 g, 4.2 mmol) in DMF (6 ml), and the mixture was stirred for 12 h. After work up and purification, 375 mg of 3 was obtained. N-tert-Butoxycarbonyl-N-3-hydroxypropyl-4-pyridylamine 4: To a solution of 3 (375 mg, 1.0 mmol) in tetrahydrofuran (THF) (10 ml) was added acetic acid (175 μl, 3.1 mmol) and 1 M tetrabutylammonium fluoride in THF (3.1 ml, 3.1 mmol) at room temperature, and the mixture was stirred for 3 h. After work up and purification, 213 mg of 4 was obtained. N-tert-Butoxycarbonyl-N-3-iodopropyl-4-pyridylamine 5: 4 (212 mg, 0.84 mmol) in CH2Cl2 (6 ml) was treated with triphenylphosphine (550 mg, 2.1 mmol), iodine (426 mg, 1.7 mmol), and imidazole (143 mg, 2.1 mmol) at 0°C for 0.5 h. After work up and purification, 272 mg of 5 was obtained. Ethyl 5-(N-tert-butoxycarbonyl-N-4-pyridylamino)-2-(3′-cyano-3-biphenylyl)pentanoate 6: After ethyl 3′-cyanophenyl-3-phenylacetate (295 mg, 1.1 mmol) in THF (3 ml) was added dropwise to a mixture of 1 M lithium bis(trimethylsilyl) amide in THF (1.16 ml, 1.2 mmol) and hexamethylphosphoramide (750 μl, 4.3 mmol) in THF (3 ml) and stirred for 0.5 h at −78°C, 5 in THF (4 ml) was added to the mixture, and the reaction mixture was allowed to reach room temperature. After work up and purification, 153 mg of 6 was obtained. Ethyl 2-(3′-amidino-3-biphenylyl)-5-(4-pyridylamino)pentanoate dihydrochloride 7: After treating 6 (40 mg, 0.08 mmol) in ethanol (1 ml) with 4 M HCl–dioxane (10 ml) at room temperature for 2 days, the mixture was concentrated. The residue was dissolved in ethanol (10 ml) and bubbled with NH3 gas until saturation at 0°C. After stirring at room temperature for 3 days, the reaction mixture was concentrated in vacuo. The residue was purified to give 25 mg of 7. FX-2212, 2-(3′-amidino-3-biphenylyl)-5-(4-pyridylamino)pentanoic acid dihydrochloride 8: 7 (20 mg, 0.04 mmol) was dissolved in 2 M HCl (4 ml), and the mixture was refluxed for 2 h. After concentration and purification, 15 mg of FX-2212 was obtained as white solids. The identity of the compound was checked by NMR and MS.