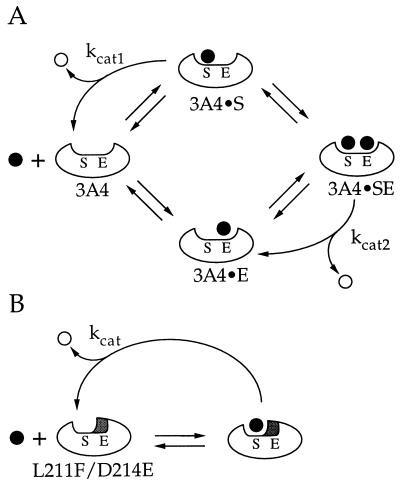
Figure 5.
(A) Model showing cooperativity of P450 3A4-catalyzed steroid hydroxylation. The enzyme active site is proposed to contain a substrate (S) and effector (E) binding site. Product (○) formation can result from substrate (•) bound in the S-site (3A4•S or 3A4•SE), but not when bound in the E-site (3A4•E). (B) Model showing reactions catalyzed by L211F/D214E. Substitution of Phe and Glu for residues Leu-211 and Asp-214, respectively, occupies a portion of the active site that normally binds effector. This renders the mutant (L211F/D214E) functionally equivalent to the 3A4•E complex in A.