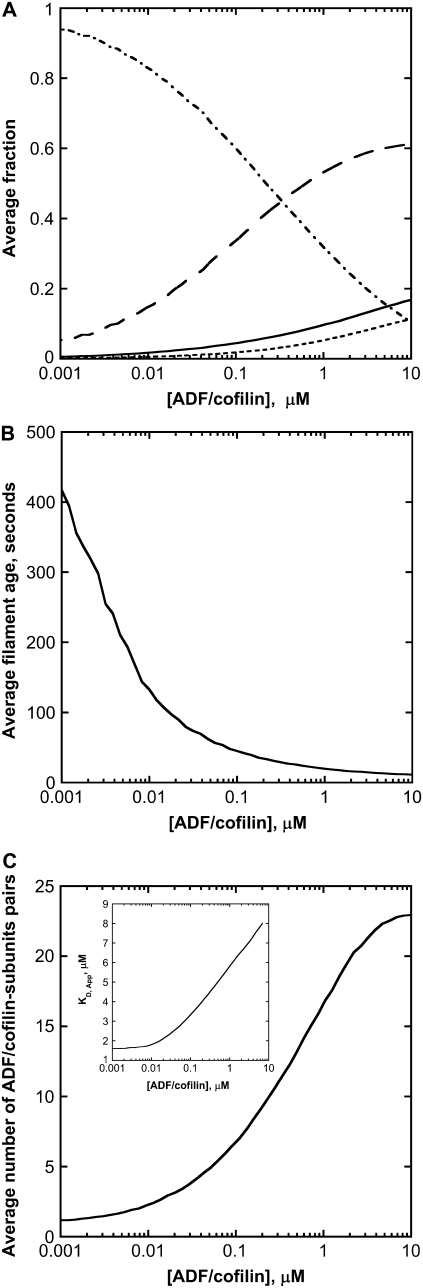
FIGURE 3.
Chemical composition of actin filaments at steady state. (A) ADF/cofilin concentration controls the molar fraction of the nucleotide bound to subunits in filament: F-ATP (solid thick curve), F-ADP-Pi (long dashed curve), F-ADP (dot-dashed curve), and F-ADP-ADF/cofilin (dotted curve). The removal of a large piece made of F-ADP subunits favors the molar ratio ATP or ADP-Pi versus ADP and ADP-ADF/cofilin-bound subunits. (B) The age of the filament decreases with ADF/cofilin activity. The age of a particular subunit in an actin filament at time t is the time spent by this subunit since its polymerization in the filament before time t. The filament age is determined by averaging the subunit ages in a filament. (C) The number of ADF/cofilin decorated subunit pairs depends on the ADF/cofilin concentration. The number of ADF/cofilin-decorated subunit pairs is a sigmoidal function of the ADF/cofilin concentration, which plateaus at high ADF/cofilin level. (Inset) Variation of the apparent dissociation equilibrium constant, KD,App for the binding of ADF/cofilin to actin filaments. For concentrations of ADF/cofilin below 0.01 μM, the KD,App is low and almost constant. Because high ADF/cofilin levels favor low F-ADP molar ratio in filaments, KD,App increases linearly with ADF/cofilin concentration. At concentrations of ADF/cofilin higher than 50 μM, KD,App plateaus at a value of 20 μM (data not shown). Parameters for simulations are listed in the legend of Fig. 2 B.