TABLE 1.
Binding of heparin to diverse CPCs
| ITC
|
DLS
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Ligand and charge (zCPC) at given pH* | Kd,i (nM)† | n binding sites per heparin‡ (nligands/nheparin) | n × zCPC | Kd,1 (nM)§ | Reaction enthalpy 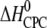 (kcal/mol CPC) (kcal/mol CPC) |
Entropy 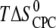 (kcal /mol CPC) (kcal /mol CPC) |
Heat capacity change¶ (cal/mol CPC/K) (cal/mol CPC/K) |
Rh** (nm) | FWHM†† of Rh (nm) |
| HIV Tat-PTD8+ | 443 ± 178 | 6.68 ± 0.57 | 53.4 ± 4.55 | 67.9 ± 30.5 | −12.3 ± 0.33 | −3.47 ± 0.37 | 38.6 | 94.4 ± 5.9 | 36.1 ± 6.6 |
| Penetratin7+ | 338 ± 105 | 6.79 ± 0.45 | 47.5 ± 3.18 | 50.6 ± 19.0 | −11.6 ± 0.12 | −2.63 ± 0.18 | −128 | 129 ± 18.7 | 51.1 ± 8.4 |
| Nona-l-arginine9+ | 459 ± 115 | 5.38 ± 0.62 | 48.4 ± 5.54 | 87.9 ± 33.3 | −12.8 ± 2.65 | −4.02 ± 2.80 | 89.3 | 98.3 ± 6.2 | 36.4 ± 5.2 |
| PLL1616+ | 543 ± 253 | 2.91 ± 0.30 | 46.6 ± 4.86 | 198 ± 108 | −8.50 ± 0.11 | 0.18 ± 0.30 | 11.0 | 77.7 ± 6.8 | 24.8 ± 6.9 |
| LPEI≈20+ (2.5 kDa); pH 7.4 | 741 ± 104 | 3.36 ± 0.51 | 67.3 ± 10.2 | 224 ± 52.1 | −16.5 ± 2.12 | −8.08 ± 2.20 | −235 | 131 ± 14.1 | 28.9 ± 7.2 |
| LPEI≈58+ (2.5 kDa); pH 5.0 | 531 ± 221 | 1.10 ± 0.27 | 64.0 ± 15.9 | 467 ± 94.9 | −37.1 ± 4.98 | −28.4 ± 4.1 | −76.1 | 123 ± 12.2 | 38.1 ± 4.1 |
| DOTAP1+ (as 30-nm small unilamellar vesicles; nDOTAP: nDOPC = 3:7) | 1345 ± 417 | 31.5 ± 3.09 | 31.5 ± 3.09 | 45.7 ± 19.0 | −0.49 ± 0.07 | 7.82 ± 0.53 | −25.7 | 586 ± 149 | 428 ± 136 |
| Acridine orange1+ | 868 ± 293 | 65.7 ± 6.8 | 65.7 ± 6.8 | 13.3 ± 4.6 | −3.82 ± 0.27 | 4.59 ± 0.48 | −32.0 | 311 ± 56.7 | 234 ± 92.5 |
| Ethidium bromide1+ | No binding observed‡‡ | <10 | |||||||
| Propidium iodide2+ | No binding observed | <10 | |||||||
| l-arginine1+ | No binding observed | <10 | |||||||
Conditions and pH (7.4) as described in Fig. 2; LPEI was measured additionally at pH 5.00 (133 mM NaCl, 30 mM acetate). Results are reported as mean ± SD from four individual sample preparations.
Microscopic dissociation constant (Kd,i) of n individual binding sites found per heparin chain.
Stoichiometry as experimentally determined (ITC) for 100% of heparin's multiple binding sites saturated with the specific ligand; heparin had a sulfur content of 11.3%, yielding an average of 45.8 sulfate groups or a total of 68.2 negative charges (including carboxyl groups) per heparin (average mol wt 13,000).
Macroscopic dissociation constant (Kd,1) for the first accessible binding site in the GAG.
Molar heat capacity change at constant pressure (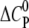 ) as calculated from the slope of
) as calculated from the slope of 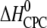 as a function of different temperatures (8, 18, 28, 38, 48, and 58°C).
as a function of different temperatures (8, 18, 28, 38, 48, and 58°C).
Hydrodynamic radius (Rh) as experimentally determined for 50% of heparin's binding sites (13.7 μM, 1.4 ml) saturated with the ligand; except for AOR and DOTAP, which were measured at 10% and 5% saturation of heparin, respectively, because at higher molar ratios, Rh exceeded the laser's wavelength. At current settings, the lower detection limit was ∼10 nm.
Width of the Rh distribution was calculated as full width at half-maximum (FWHM).
Physiological ionic strength apparently prevents binding of ethidium bromide to heparin (32).
