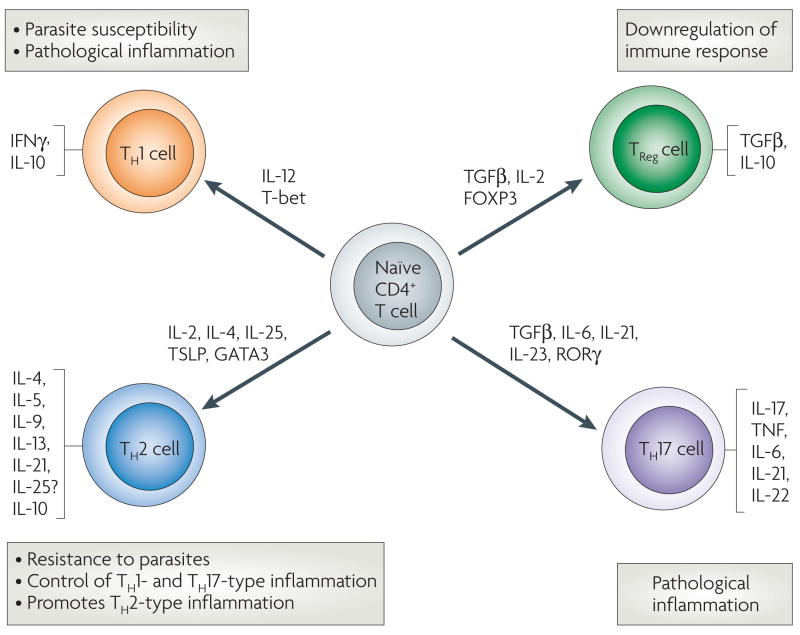
Figure 4. T helper and regulatory cells.
Naive CD4+ T cells can differentiate into several different types of effector and regulatory cells during helminth infection. Specific cytokines and transcription factors contribute to differentiation and expansion of these cell populations, and their differential activation plays a major role in determining whether an immune response will contribute to host protection or pathological inflammation. Exposure to interleukin-12 (IL-12) produced by antigen-presenting dendritic cells (DCs) induces T-bet expression, driving T helper 1 (TH1)-cell differentiation. These cells produce interferon-γ (IFNγ), and are unable to clear helminth parasites. The circumstances leading to TH2-cell differentiation are not as clear but recently, IL-2, IL-25 and thymic stromal lymphopoietin (TSLP), and associated transcription factors, have been implicated. TH2 cells produce a panel of cytokines that contribute to antihelminth immunity by instructing innate bone-marrow- and non-bone-marrow-derived cells, which in turn can instruct and amplify TH2 cells. Strong TH2-type responses often lead to increased resistance to parasites, as seen in Heligmosomoides polygyrus infection, or control of harmful inflammation mediated by TH1 cells and TH17 cells, as in schistosomiasis. However, chronic potent TH2-cell responses can also lead to harmful TH2-type inflammatory responses including fibrosis. Expression of the transcription factor forkhead box P3 (FOXP3) along with the cytokines transforming growth factor-β (TGFβ) and IL-2, leads to differentiation of regulatory T cells (TReg cells), which can inhibit the immune response. Several factors, including retinoic-acid-receptor-related orphan receptor-γ (RORγ), TGFβ, IL-6, IL-21 and IL-23, have been shown to promote TH17-cell differentiation, and these effector cells can mediate harmful inflammation. IL-10, which is no longer considered to be exclusively a TH2-type cytokine, can be produced by TH1, TH2, and TRegcells and downregulates both TH1- and TH2-type responses. TNF, tumour-necrosis factor.